 全部商品分类
全部商品分类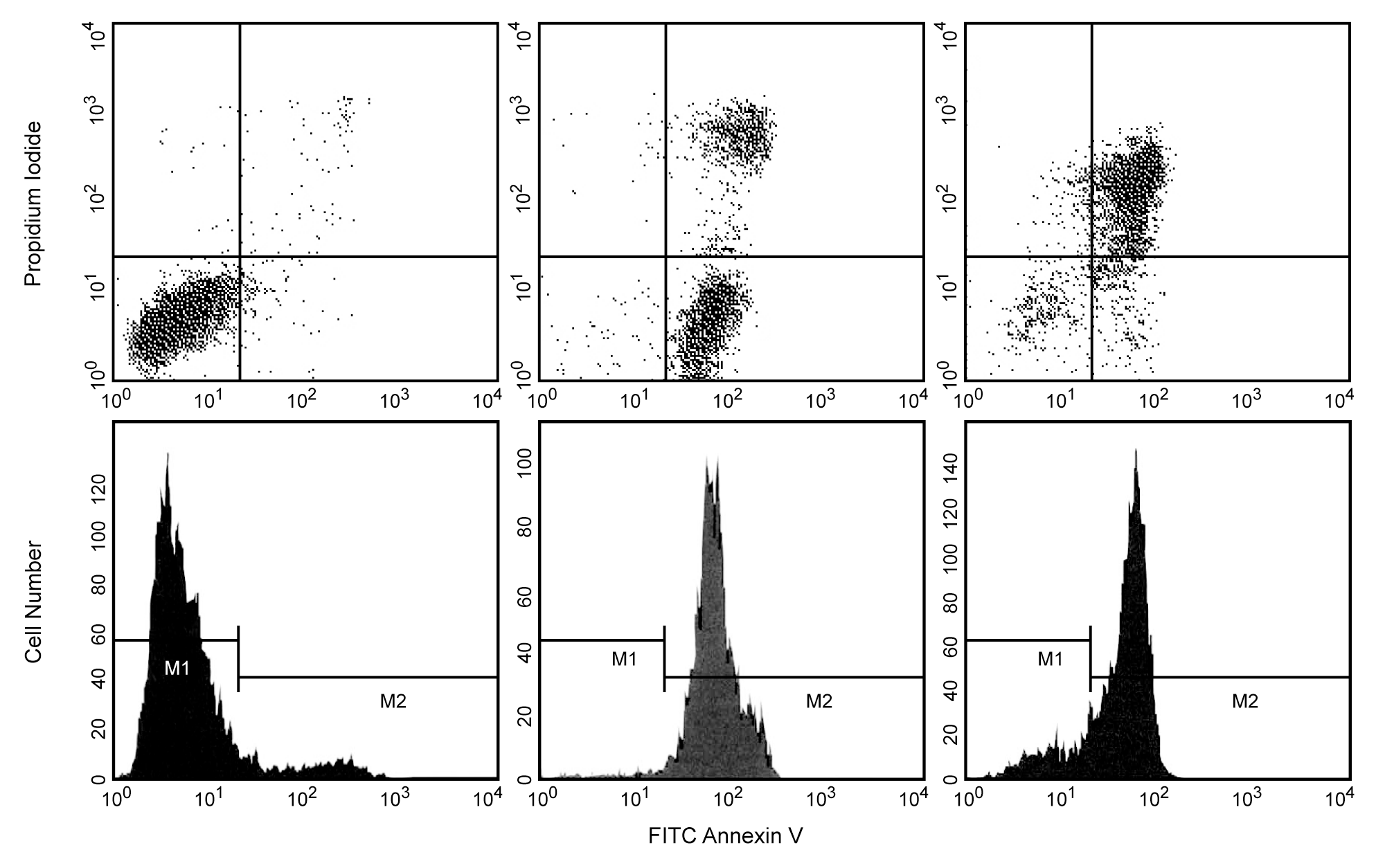










参考图片
FITC Annexin V: A tool for identifying cells that are undergoing apoptosis. HBP-ALL human leukemia cells were left untreated (top left & bottom left panels), treated for 5 hr (top middle & bottom middle panels) or 12 hr (top right & bottom right panels) with anti-human Fas antibody (Clone DX2, Cat. No. 555670) and Protein G. Cells were incubated with FITC Annexin V in a buffer containing propidium iodide (PI) and analyzed by flow cytometry. Untreated cells were primarily FITC Annexin V and PI negative, indicating that they were viable and not undergoing apoptosis. After a 5 hr treatment with DX2, the majority of the cells were either undergoing apoptosis (FITC Annexin V positive and PI negative) or had already died (FITC Annexin V and PI positive). After a 12 hr treatment with DX2, the majority of the cells had already died (FITC Annexin V and PI positive). The addition of Protein G enhances the ability of DX2 to induce apoptosis, presumably by cross-linking the Fas receptor.
FITC Annexin V: A tool for identifying cells that are undergoing apoptosis. HBP-ALL human leukemia cells were left untreated (top left & bottom left panels), treated for 5 hr (top middle & bottom middle panels) or 12 hr (top right & bottom right panels) with anti-human Fas antibody (Clone DX2, Cat. No. 555670) and Protein G. Cells were incubated with FITC Annexin V in a buffer containing propidium iodide (PI) and analyzed by flow cytometry. Untreated cells were primarily FITC Annexin V and PI negative, indicating that they were viable and not undergoing apoptosis. After a 5 hr treatment with DX2, the majority of the cells were either undergoing apoptosis (FITC Annexin V positive and PI negative) or had already died (FITC Annexin V and PI positive). After a 12 hr treatment with DX2, the majority of the cells had already died (FITC Annexin V and PI positive). The addition of Protein G enhances the ability of DX2 to induce apoptosis, presumably by cross-linking the Fas receptor.








 用小程序,查商品更便捷
用小程序,查商品更便捷




