 全部商品分类
全部商品分类

 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷


 收藏
收藏
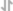 对比
对比 咨询
咨询




参考图片
Panel 1. Two-color flow cytometric analysis of Jurkat cell viability. Jurkat cells were treated with 5 µM Camptothecin (Right Plot) or DMSO vehicle (Left Plot) overnight. Cells were resuspended in Annexin V Binding Buffer (Cat. No. 556454) and stained with FITC Annexin V (Cat. No. 556419) and 1.25 µM DRAQ7™ (Cat. No. 564904). Camptothecin-treated cells show an increased frequency of apoptotic (Annexin V+ DRAQ7™-) and dead (Annexin V+ DRAQ7™+) cells. Analysis was performed using a BD LSRFortessa™ Cell Analyzer System. Panel 2. Flow cytometric analysis of HeLa cell DNA content. Cultured HeLa cells in log phase growth were harvested using Gibco® Cell Dissociation Buffer (Life Technologies), fixed, and permeabilized using the BD Pharmingen™ Transcription Factor Buffer Set (Cat. No. 562574/562725). Cells were resuspended in DPBS with 20 μM DRAQ7™ and analyzed using a low BD LSRFortessa™ cytometer flow rate. Histograms were deconvoluted by FlowJo™ software into G0/G1, S, and G2/M populations. Panel 3. Immunoflourescent staining of Alkaline Phosphatase on human embryonic stem (ES) cells. H9 human ES cells (WiCell, Madison, WI) passage 44 were cultured on mTeSR™1 medium (StemCell Technologies) and fixed with BD Cytofix™ fixation buffer (Cat. No. 554655). The fixed cells were stained with Alexa Fluor® 488 Mouse anti-Human Alkaline Phosphatase monoclonal antibody (pseudo-colored green, Cat. No. 561495). And 5 μM DRAQ7™ (pseudo-colored red) was used as a nuclear counterstain. The images were captured on a BD Pathway™ 435 Cell Analyzer and merged using BD Attovision™ Software. DRAQ7™ was also tested in mouse (data not shown).





