全部商品分类
全部商品分类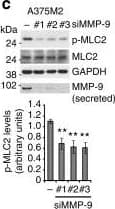



 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷


 收藏
收藏
 对比
对比 咨询
咨询Immunohistochemistry(8-25 µg/mL)
Immunoprecipitation(25 µg/mL)



Scientific Data
 View Larger
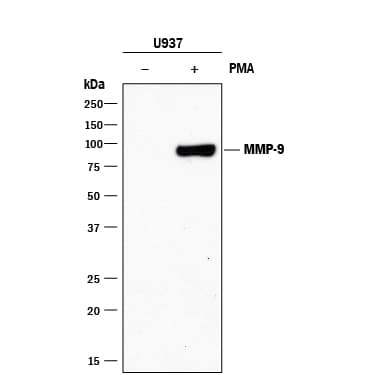
View LargerDetection of Human MMP‑9 by Western Blot. Western blot shows lysates of U937 human histiocytic lymphoma cell line untreated (-) or treated (+) with 5 ng/mL PMA for 24 hours. PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human MMP-9 Monoclonal Antibody (Catalog # MAB911) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for MMP-9 at approximately 85 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
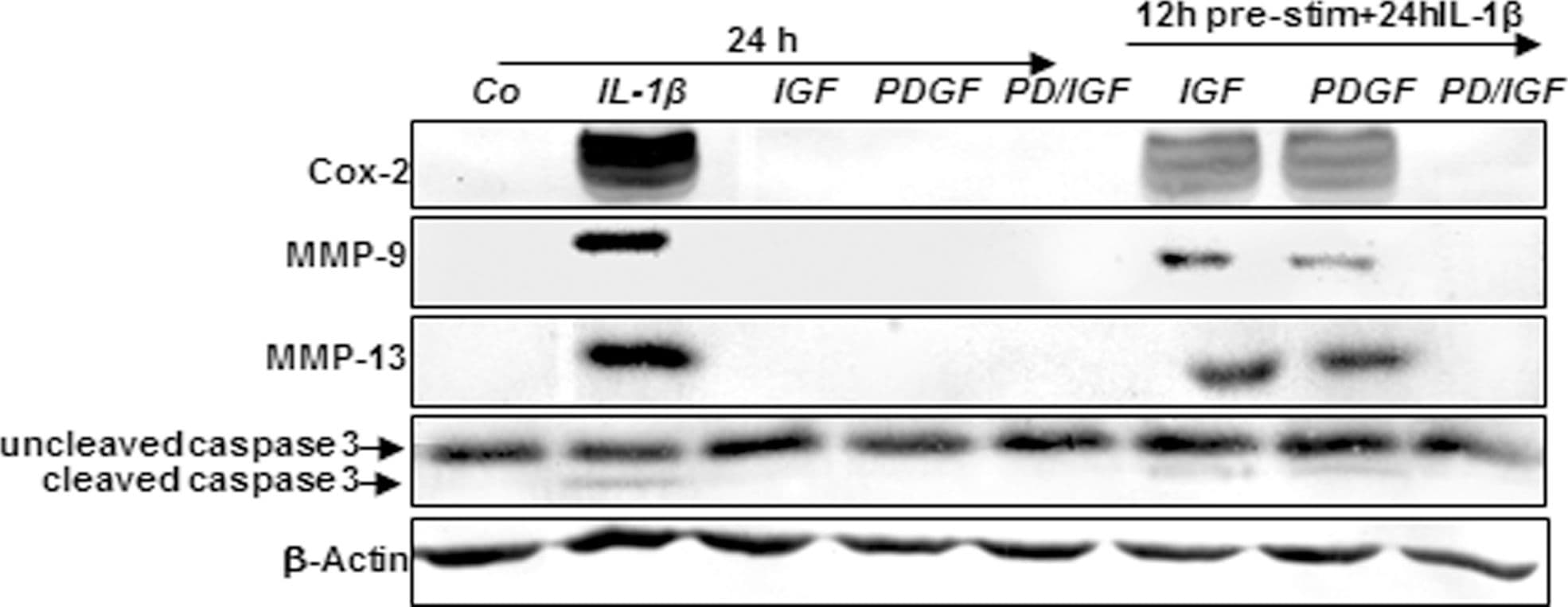
View LargerDetection of Canine MMP-9 by Western Blot Effects of IGF-1 or/and PDGF-bb on IL-1 beta -induced NF-kappa B-dependent pro-inflammatory, pro-apoptotic and matrix degrading gene products in chondrocytes.To determine whether IGF-1 or/and PDGF-bbexert effects on IL-1 beta -induced NF-kappa B-dependent expression of pro-inflammatory, pro-apoptotic and matrix degrading gene products, primary chondrocytes were either stimulated with 10 ng/ml IL-1 beta, 10 ng/ml PDGF-bb, 10 ng/ml IGF-1 or combination of both growth factors (5 ng/ml each) or pre-stimulated for 12 h with 10 ng/ml PDGF-bb, 10 ng/ml IGF-1 or combination of both growth factors (5 ng/ml each) followed by 10 ng/ml IL-1 beta for 24. Equal amounts of total proteins were separated by SDS-PAGE and analyzed by immunoblotting using antibodies raised against COX-2, MMP-9 and MMP-13 and active caspase-3. Stimulation with IL-1 beta resulted in production of COX-2, MMP-9, MMP-13 and caspase-3 cleavage. Pre-treatment with a combination of both IGF-1 or/and PDGF-bb downregulated COX-2, MMP-9, MMP-13 and cleaved caspase-3. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0028663), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View LargerDetection of Human MMP-9 by Knockdown Validated MMP-9 positively regulates roundness and MLC2 activity(a) Cell morphology (roundness) of A375M2 cells on top of bovine collagen I after MMP-9 knockdown (siMMP-9). Dots represent single cells from two independent experiments. Representative F-actin-staining images are shown below. Scale bar, 20 μm. (b) Representative bright-field images of A375M2 cells on top of bovine collagen I after MMP-9 knockdown. Scale bar, 50 μm. (c) Representative immunoblot (top) and phospho-MLC2 (p-MLC2) levels (bottom) of A375M2 cells on bovine collagen I after MMP-9 knockdown. MMP-9 immunoblot is also shown (n = 9). (d) Representative confocal images (top) and quantification (bottom) of p-MLC2 immunostaining in A375M2 cells on bovine collagen I after MMP-9 knockdown. Dots represent single cells from three independent experiments. Scale bar, 25 μm. (e) Representative confocal images of A375P cells on bovine collagen I treated with 2 μg ml−1 recombinant purified proMMP-9 for 24 h. MMP-9 (cyan) and F-actin (red) stainings are shown. Scale bar, 25 μm. (f) Cell morphology (roundness) of A375P cells after proMMP-9 treatment for 24 h. Dots represent single cells from three independent experiments. (g) Representative proMMP-9 immunoblot in A375P cells on bovine collagen I after treatment with 2–4 μg ml−1 proMMP-9 for 24 h. (h) Diagram representing addition of A375M2- or A375P-secreted media to A375P cells on top of bovine collagen I. (i) Percentage of A375P elongated cells (associated with loss of cell rounding) grown on bovine collagen I for 24 h in the presence of secreted media from A375P, A375M2 or A375M2 siMMP-9 cells (n = 3). (j) Representative immunoblot (left) and p-MLC2 levels (right) in A375P cells on bovine collagen I for 24 h in the presence of secreted media from A375P, A375M2 or A375M2 siMMP-9 cells. MMP-9 immunoblot is also shown (n = 3). Graphs show mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. ANOVA with Tukey’s post hoc test (a,c,d,i,j), unpaired t-test (f). Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/ncomms5255), licensed under a CC-BY license. Not internally tested by R&D Systems.
Human MMP-9 Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Immunohistochemistry(8-25 µg/mL)
Immunoprecipitation(25 µg/mL)


Background: MMP-9
Matrix metalloproteinases are a family of zinc and calcium dependent endopeptidases with the combined ability to degrade all the components of the extracellular matrix. MMP-9 (Gelatinase B) can degrade a broad range of substrates including gelatin, collagen types IV and V, elastin and proteoglycan core protein. It is believed to act synergistically with interstitial collagenase (MMP-1) in the degradation of fibrillar collagens as it degrades their denatured gelatin forms. MMP-9 is produced by keratinocytes, monocytes, macrophages and PMN leukocytes. MMP-9 is present in most cases of inflammatory responses. Structurally, MMP-9 maybe be divided into five distinct domains: a pro-domain which is cleaved upon activation, a gelatin-binding domain consisting of three contiguous fibronectin type II units, a catalytic domain containing the zinc binding site, a proline-rich linker region, and a carboxyl terminal hemopexin-like domain.


Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
参考图片
Detection of Human MMP‑9 by Western Blot. Western blot shows lysates of U937 human histiocytic lymphoma cell line untreated (-) or treated (+) with 5 ng/mL PMA for 24 hours. PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human MMP‑9 Monoclonal Antibody (Catalog # MAB911) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018).
A specific band was detected for MMP‑9 at approximately 85 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.