 全部商品分类
全部商品分类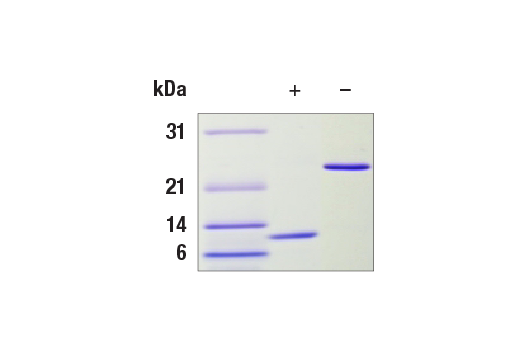


Recombinant human TGF-β1 was expressed in CHO cells and is supplied in a lyophilized form.


| MW (kDa) | 25.6 |
| Purity | A greater than or equal to 95% purity was determined by SDS-PAGE. |
| Endotoxin | Endotoxin levels are less than or equal to 1 EU / 1 μg hTGF-β1. |
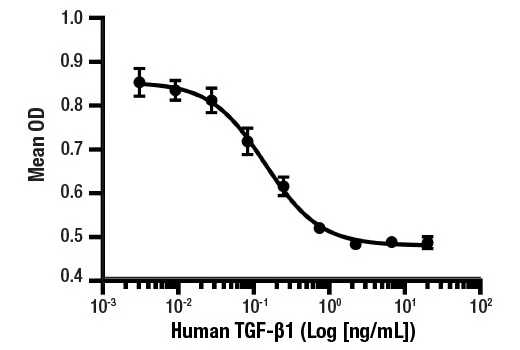
| Activity | The bioactivity of recombinant hTGF-β1 was determined in an IL-4 induced HT-2 cell proliferation assay. The ED50 of each lot is less than or equal to 0.5 ng/mL. |







Human TGF-β1 Recombinant Protein is supplied as lyophilized material that is very stable at -20°C. It is recommended to reconstitute with sterile 10 mM HCl at a concentration of 0.1 mg/mL which can be further diluted in aqueous solutions as needed. Addition of a carrier protein (0.1% HSA or BSA) is recommended for long-term storage.
Once in solution, store at 4°C and use within 1 month, or store at -20ºC to -80ºC and use within 3 months to prevent loss of potency. Aliquot to avoid multiple freeze/thaw cycles if storing reconstituted material at -20ºC to -80ºC.


参考图片
The purity of Human TGF-β1 Recombinant Protein was determined by SDS-PAGE of 1 µg reduced (+) and non-reduced (-) recombinant hTGF-β1 and staining with Coomassie Blue. hTGF-β1 is a homodimer with a predicted total molecular weight (MW) of 25.6 kDa with each subunit equaling 12.8 kDa.
Serial dilutions of Human TGF-β1 Recombinant Protein were added to HT-2 cells. Inhibition of IL-4 induced cell proliferation was measured and the linear portion of the curve was used to calculate the ED50.





 用小程序,查商品更便捷
用小程序,查商品更便捷




