全部商品分类
全部商品分类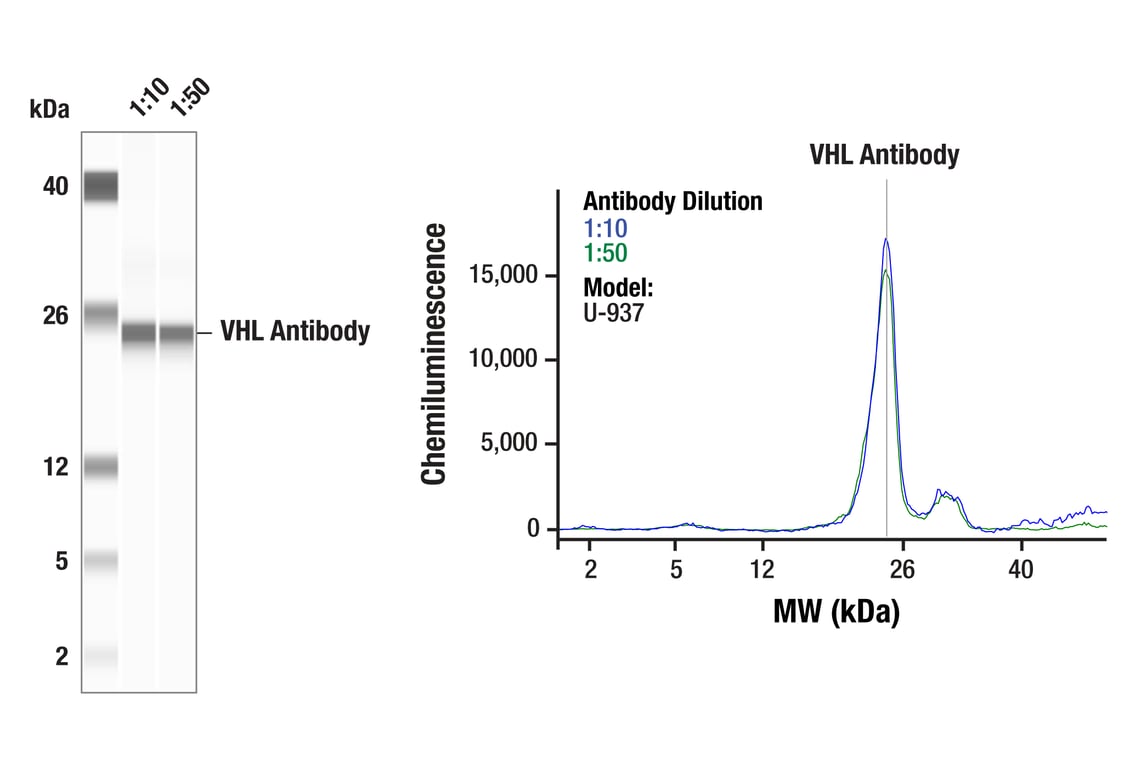



 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷


 收藏
收藏
 对比
对比 咨询
咨询
The Hypoxia Pathway Antibody Sampler Kit provides an economical means to investigate select proteins involved in the hypoxia pathway. The kit contains enough primary antibodies to perform two western blot experiments with each primary antibody.






参考图片
Simple Western™ analysis of lysates (0.1 mg/mL) from U-937 cells using VHL Antibody #68547. The virtual lane view (left) shows the target band (as indicated) at 1:10 and 1:50 dilutions of primary antibody. The corresponding electropherogram view (right) plots chemiluminescence by molecular weight along the capillary at 1:10 (blue line) and 1:50 (green line) dilutions of primary antibody. This experiment was performed under reducing conditions on the Jess™ Simple Western instrument from ProteinSimple, a BioTechne brand, using the 2-40 kDa separation module.
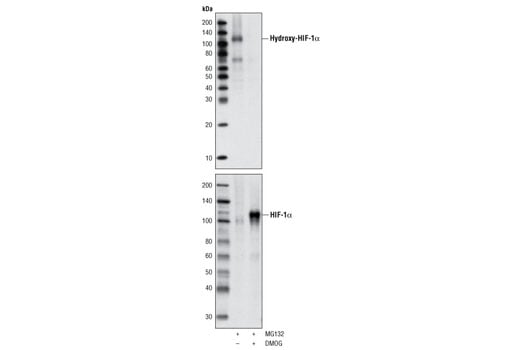
Western blot analysis of extracts from HeLa cells, treated with either 10 μM of MG132 (to accumulate hydroxylated HIF-1α) or 10 µM MG132 and 1 mM DMOG (to accumulate nonhyroxylated HIF-1α), using Hydroxy-HIF-1α (Pro564) (D43B5) XP® Rabbit mAb (upper) or total HIF-1α Antibody #3716 (lower).
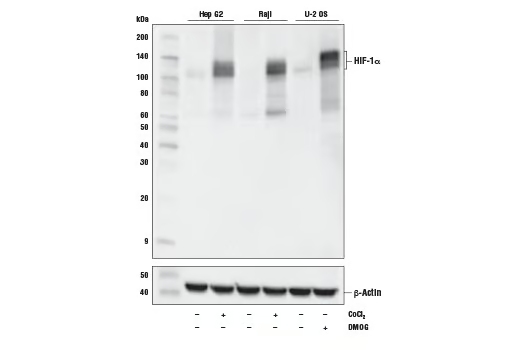
Western blot analysis of extracts from Hep G2 cells untreated (-) or treated with cobalt chloride (100 µM, 4 h; +), Raji cells untreated (-) or treated with cobalt chloride (100 µM, 4 h; +) and U-2 OS cells untreated (-) or treated with DMOG (1 mM, 6 h; +) using HIF-1α (D1S7W) XP® Rabbit mAb (upper) or β-Actin (D6A8) Rabbit mAb #8457 (lower).
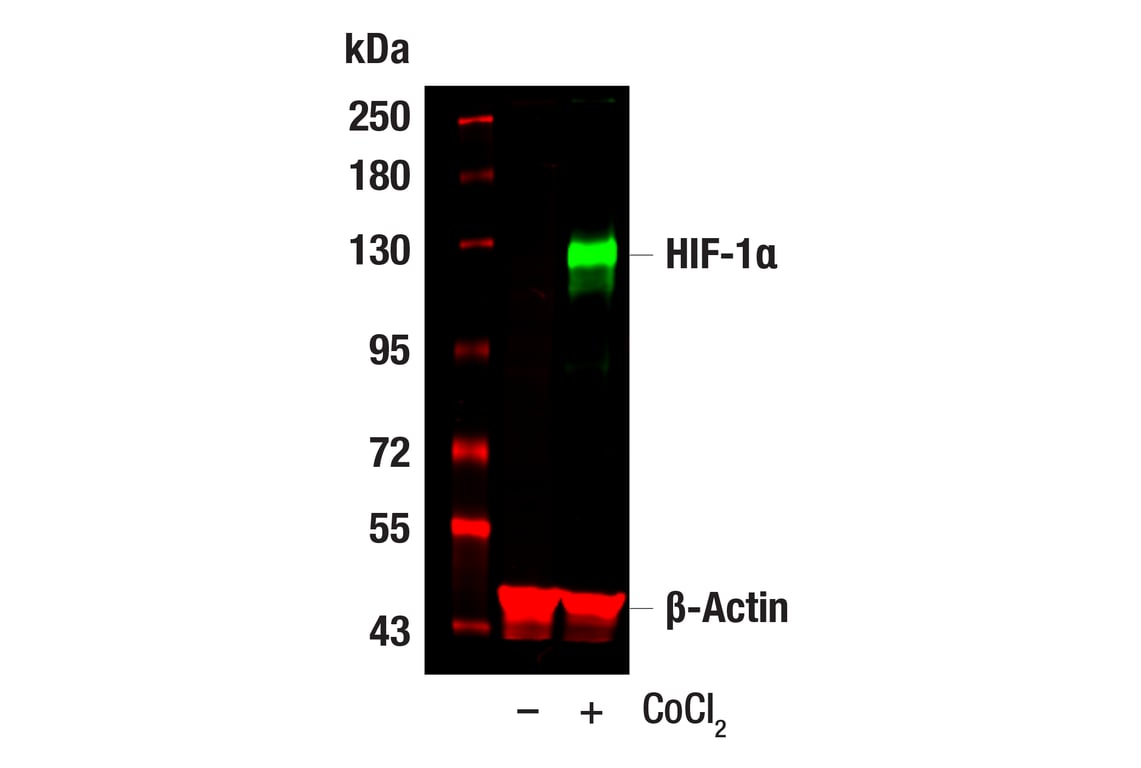
Western blot analysis of extracts from Hep G2 cells, untreated (-) or treated with cobalt chloride (100 μM, 24 hr; +), using HIF-1α (D1S7W) XP® Rabbit mAb #36169 (green), and β-Actin (8H10D10) Mouse mAb #3700 (red). Anti-rabbit IgG (H+L) (DyLight 800 4X PEG Conjugate) #5151 (green) and Anti-mouse IgG (H+L) (DyLight 680 Conjugate) #5470 (red) were used as secondary antibodies.
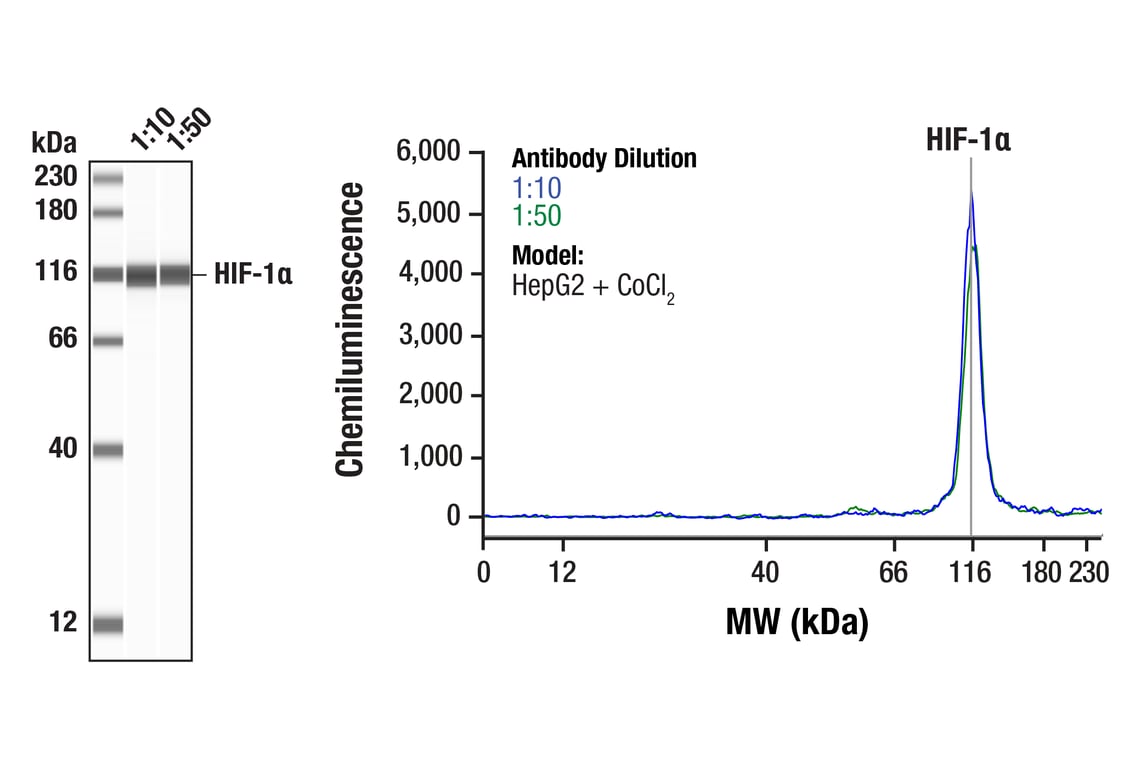
Simple Western™ analysis of lysates (0.1 mg/mL) from HepG2 cells treated with cobalt chloride (100 µM, 24 hr) using HIF-1α (D1S7W) XP® Rabbit mAb #36169. The virtual lane view (left) shows the target band (as indicated) at 1:10 and 1:50 dilutions of primary antibody. The corresponding electropherogram view (right) plots chemiluminescence by molecular weight along the capillary at 1:10 (blue line) and 1:50 (green line) dilutions of primary antibody. This experiment was performed under reducing conditions on the Jess™ Simple Western instrument from ProteinSimple, a BioTechne brand, using the 12-230 kDa separation module.
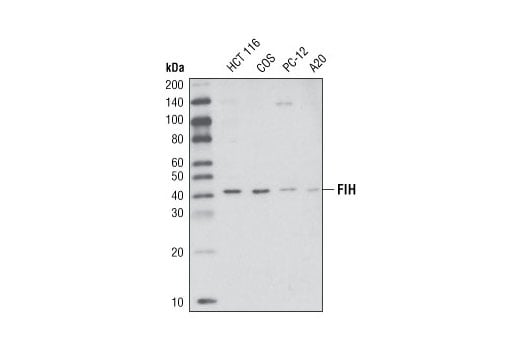
Western blot analysis of extracts from various cell types using FIH (D19B3) Rabbit mAb.
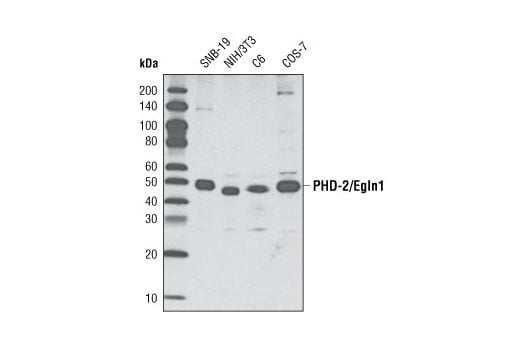
Western blot analysis of extracts from various cell types using PHD-2/Egln1 (D31F11) Rabbit mAb.
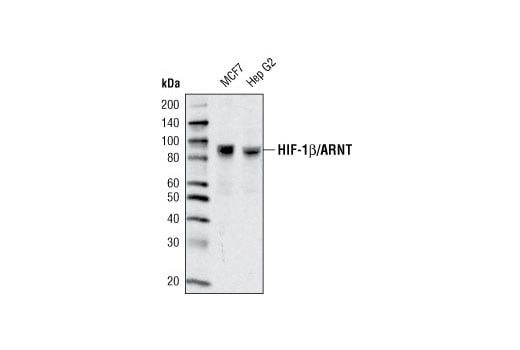
Western blot analysis of extracts from various cell types using HIF-1β/ARNT (D28F3) XP® Rabbit mAb.
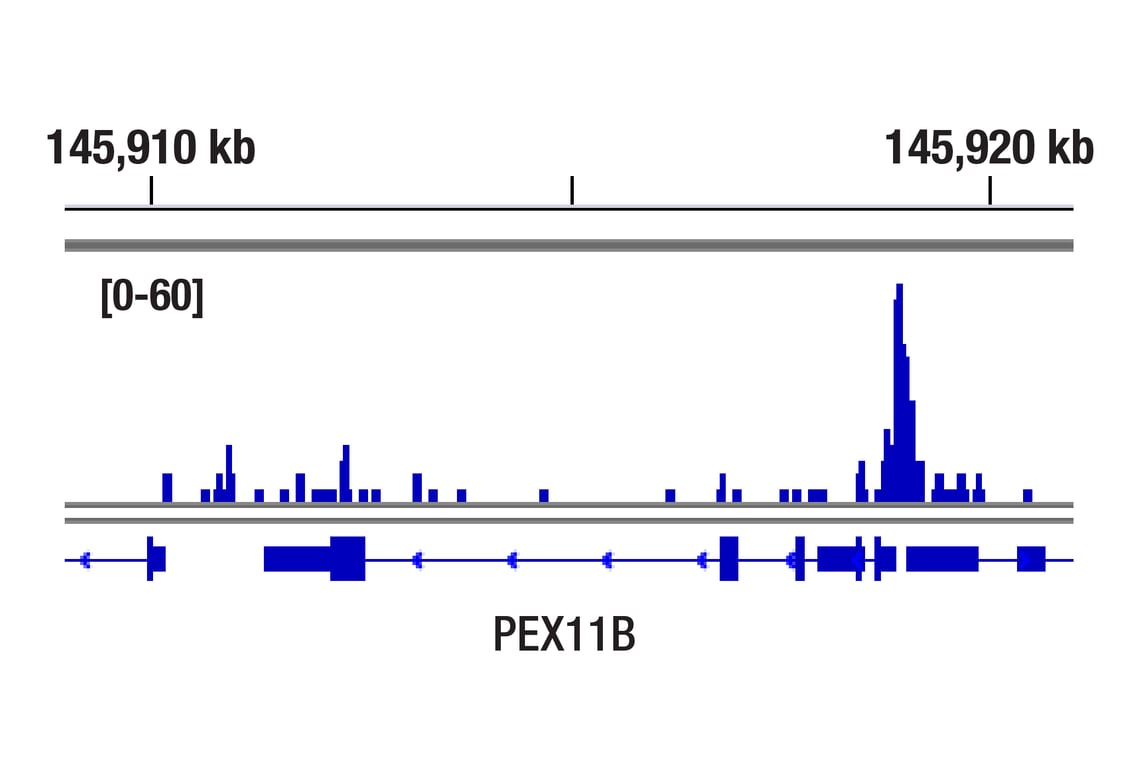
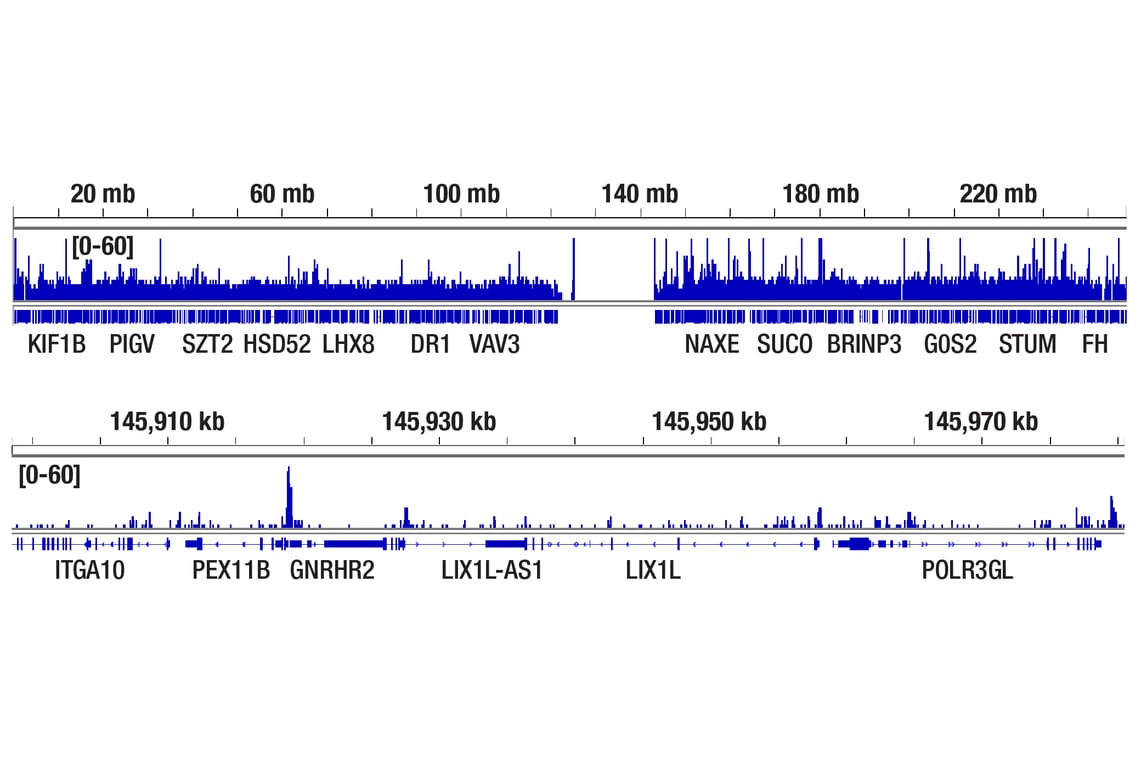
CUT&RUN was performed with T47D cells treated with BNF (1μM) for 45 min and HIF-1β/ARNT (D28F3) XP® Rabbit mAb, using CUT&RUN Assay Kit #86652. DNA library was prepared using DNA Library Prep Kit for Illumina Systems (ChIP-seq, CUT&RUN) #56795. The figure shows binding across PEX11B gene.
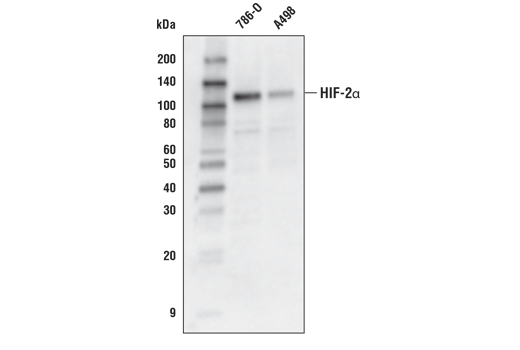
Western blot analysis of extracts from 786-O and A498 cells using HIF-2α (D6T8V) Rabbit mAb.
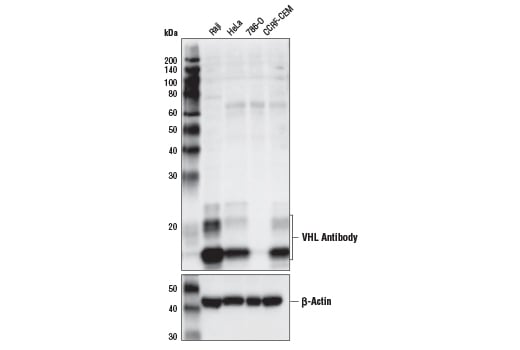
Western blot analysis of extracts from various cell lines using VHL Antibody (upper) and β-Actin (D6A8) Rabbit mAb #8457 (lower). Note that 786-O is a VHL-null cell line, demonstrating specificity of the antibody.
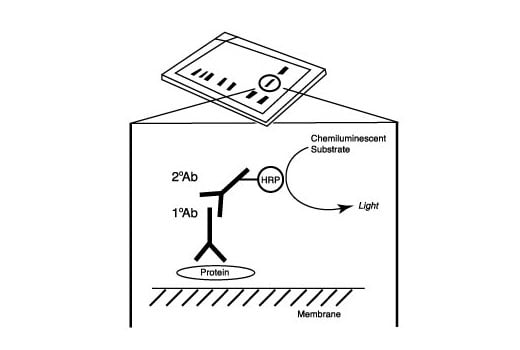
After the primary antibody is bound to the target protein, a complex with HRP-linked secondary antibody is formed. The LumiGLO® is added and emits light during enzyme catalyzed decomposition.
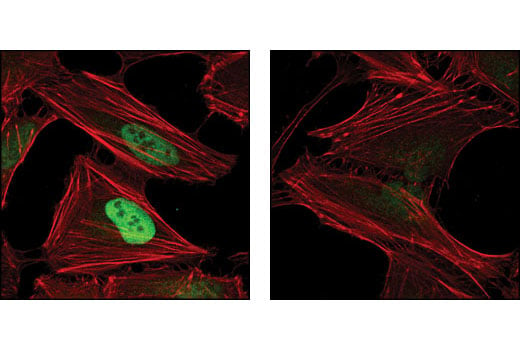
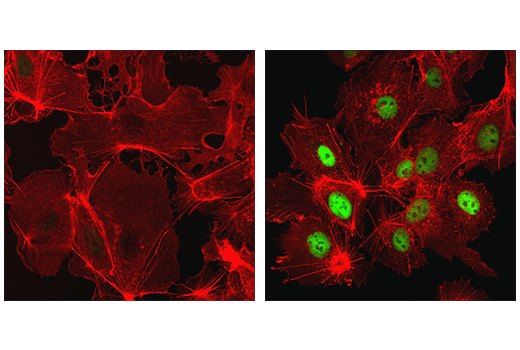
Confocal immunofluorescent analysis of HeLa cells, treated with either 10 μM MG132 (left) or 10 μM MG132 and 1 mM DMOG (right), using Hydroxy-HIF-1α (Pro564) (D43B5) XP® Rabbit mAb (green). Actin filaments have been labeled using DY-554 phalloidin (red).
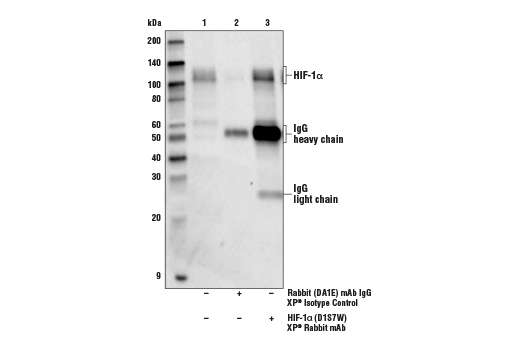
Immunoprecipitation of HIF-1α from lysate of Hep G2 cells treated with cobalt chloride (100 µM, 4 h). Lane 1 is 10% input, lane 2 is Rabbit (DA1E) mAb IgG XP® Isotype Control #3900, and lane 3 is HIF-1α (D1S7W) XP® Rabbit mAb. Western blot analysis was performed using HIF-1α (D1S7W) XP® Rabbit mAb. Anti-rabbit IgG, HRP-linked Antibody #7074 was used as the secondary antibody.
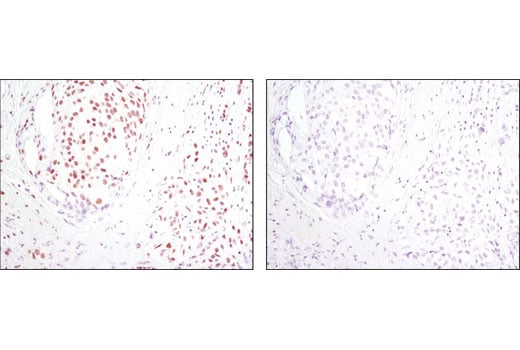
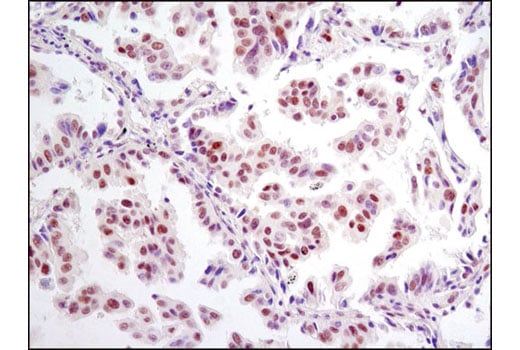
Immunohistochemical analysis of paraffin-embedded human breast carcinoma using HIF-1β/ARNT (D28F3) XP® Rabbit mAb in the presence of control peptide (left) or antigen-specific peptide (right).
CUT&RUN was performed with T47D cells treated with BNF (1μM) for 45 min and HIF-1β/ARNT (D28F3) XP® Rabbit mAb, using CUT&RUN Assay Kit #86652. DNA library was prepared using DNA Library Prep Kit for Illumina Systems (ChIP-seq, CUT&RUN) #56795. The figures show binding across chromosome 1 (upper), including PEX11B (lower).
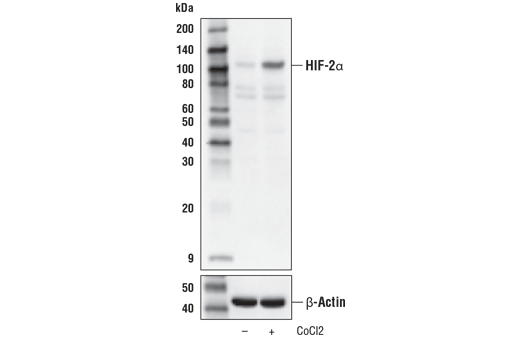
Western blot analysis of Hep G2 cells, untreated (-) or treated with cobalt chloride (CoCl2, 100 μM, 4 hr, +) using HIF-2α (D6T8V) Rabbit mAb (upper) or β-Actin (D6A8) Rabbit mAb #8457 (lower).
Confocal immunofluorescent analysis of Hep G2 cells, untreated (left) or treated with cobalt chloride (500 μM, 24 h; right), using HIF-1α (D1S7W) XP® Rabbit mAb (green). Actin filaments were labeled with DyLight™ 554 Phalloidin #13054 (red).
Immunohistochemical analysis of paraffin-embedded human lung carcinoma using HIF-1β/ARNT (D28F3) XP® Rabbit mAb.
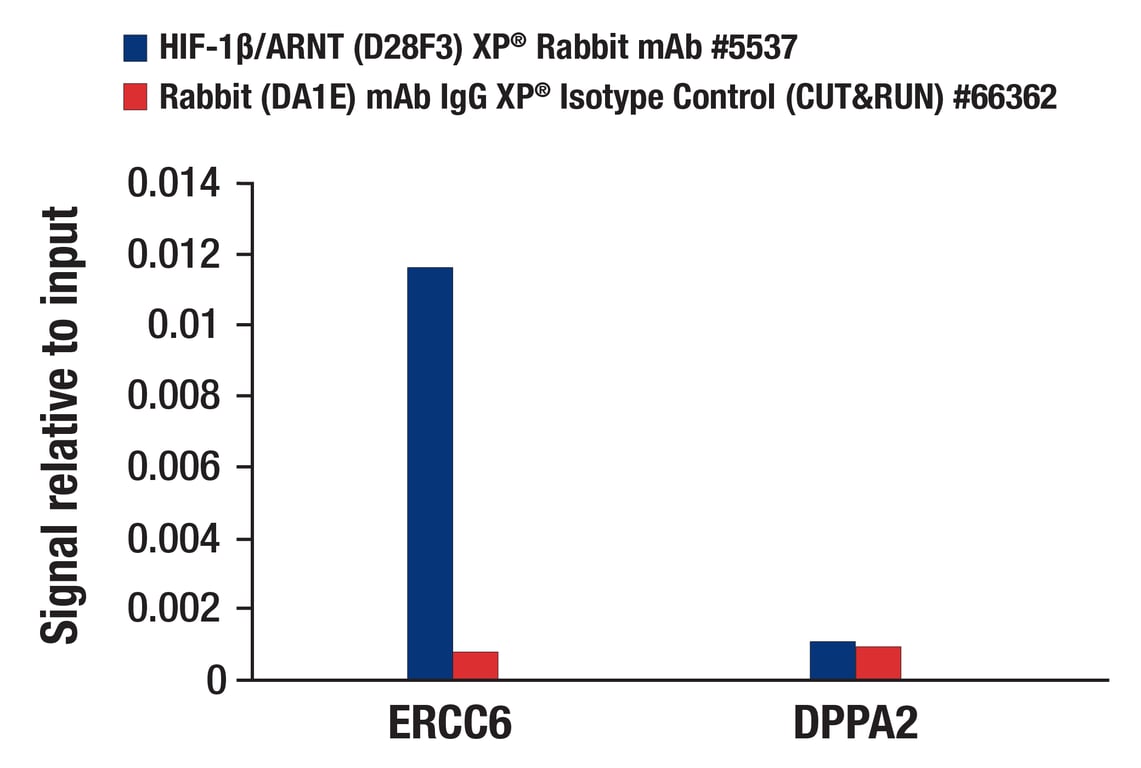
CUT&RUN was performed with T47D cells treated with BNF (1μM) for 45 min and either HIF-1β/ARNT (D28F3) XP® Rabbit mAb or Rabbit (DA1E) mAb IgG XP® Isotype Control (CUT&RUN) #66362, using CUT&RUN Assay Kit #86652. The enriched DNA was quantified by real-time PCR using human ERCC6 promoter primers and human DPPA2 intron 1 primers. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.
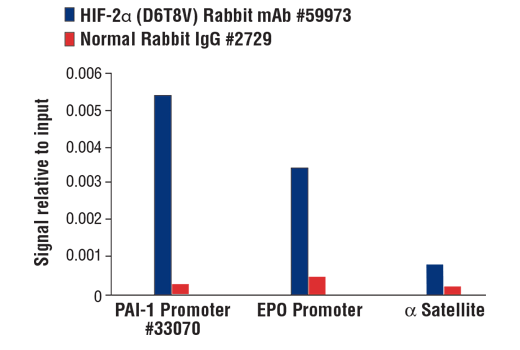
Chromatin immunoprecipitations were performed with cross-linked chromatin from Hep3B2.1-7 cells treated with cobalt chloride (100 μM) overnight and either HIF-2α (D6T8V) Rabbit mAb #59973 or Normal Rabbit IgG #2729 using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. The enriched DNA was quantified by real-time PCR using SimpleChIP® Human PAI-1 Promoter Primers #33070, human EPO promoter primers, and SimpleChIP® Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.
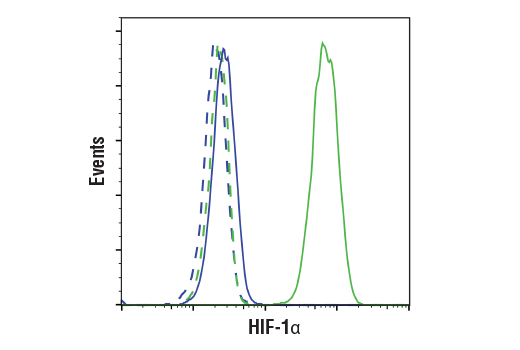
Flow cytometric analysis of U-2 OS cells, untreated (blue) or treated with DMOG (1 mM, 6 h; green), using HIF-1α (D1S7W) XP® Rabbit mAb (solid lines) or concentration-matched Rabbit (DA1E) mAb IgG XP® Isotype control #3900 (dashed lines). Anti-rabbit IgG (H+L), F(ab')2 Fragment (Alexa Fluor® 488 Conjugate) #4412 was used as a secondary antibody.
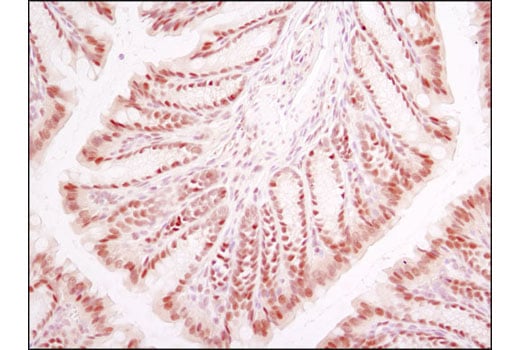
Immunohistochemical analysis of paraffin-embedded mouse colon using HIF-1β/ARNT (D28F3) XP® Rabbit mAb.
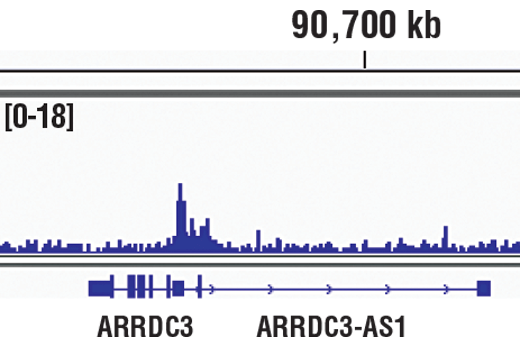
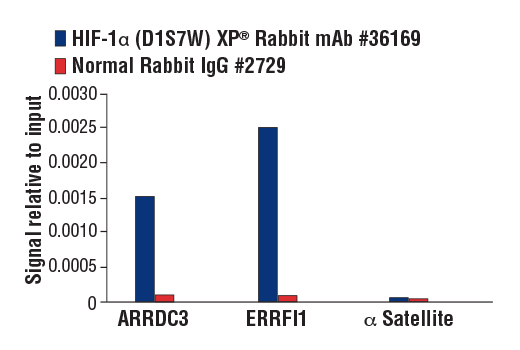
Chromatin immunoprecipitations were performed with cross-linked chromatin from MCF7 cells treated with cobalt chloride (100 μM) overnight and HIF-1α (D1S7W) XP® Rabbit mAb, using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. DNA Libraries were prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figure shows binding across ARRDC3, a known target gene of HIF-1α (see additional figure containing ChIP-qPCR data).
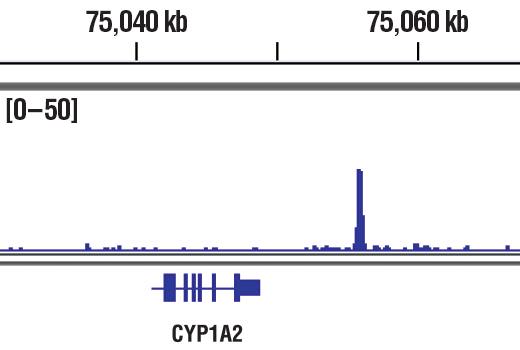
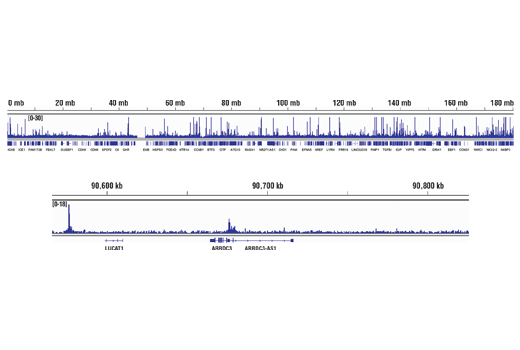
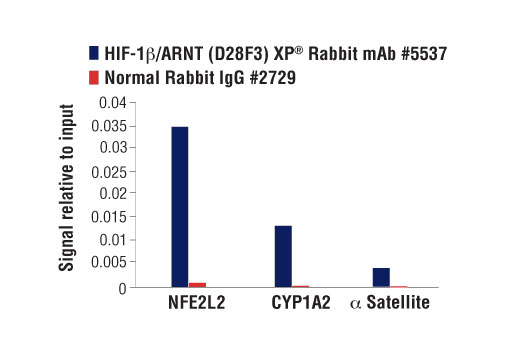
Chromatin immunoprecipitations were performed with cross-linked chromatin from T47D cells treated with BNF (1μM) for 45 min and HIF-1β/ARNT (D28F3) XP® Rabbit mAb, using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads)#9005. DNA Libraries were prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figure shows binding across CYP1A2, a known target gene of HIF-1β (see additional figure containing ChIP-qPCR data). For additional ChIP-seq tracks, please download the product data sheet.
Chromatin immunoprecipitations were performed with cross-linked chromatin from MCF7 cells treated with cobalt chloride (100 μM) overnight and HIF-1α (D1S7W) XP® Rabbit mAb, using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. DNA Libraries were prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figure shows binding across chromosome 5 (upper), including ARRDC3 (lower), a known target gene of HIF-1α (see additional figure containing ChIP-qPCR data).
Chromatin immunoprecipitations were performed with cross-linked chromatin from T47D cells treated with BNF (1μM) for 45 min and either HIF-1β/ARNT (D28F3) XP® Rabbit mAb or Normal Rabbit IgG #2729 using SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) #9003. The enriched DNA was quantified by real-time PCR using SimpleChIP® Human NFE2L2 Intron 1 Primers #81126, human CYP1A1 promoter primers, and SimpleChIP® Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.
Chromatin immunoprecipitations were performed with cross-linked chromatin from MCF7 cells treated with cobalt chloride (100 μM, overnight) and either HIF-1α (D1S7W) XP® Rabbit mAb or Normal Rabbit IgG #2729, using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. The enriched DNA was quantified by real-time PCR using human ARRDC3 downstream primers, SimpleChIP® Human ERRFI1 Upstream Primers #31180, and SimpleChIP® Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.
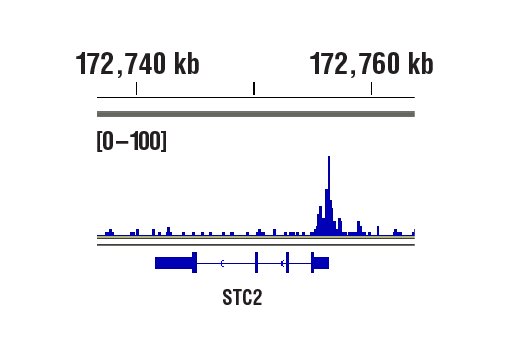
CUT&RUN was performed with MCF7 cells treated with cobalt chloride (100 μM) overnight and HIF-1α (D1S7W) XP® Rabbit mAb, using CUT&RUN Assay Kit #86652. DNA library was prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figure shows binding across STC2, a known target gene of HIF-1α (see additional figure containing CUT&RUN-qPCR data).
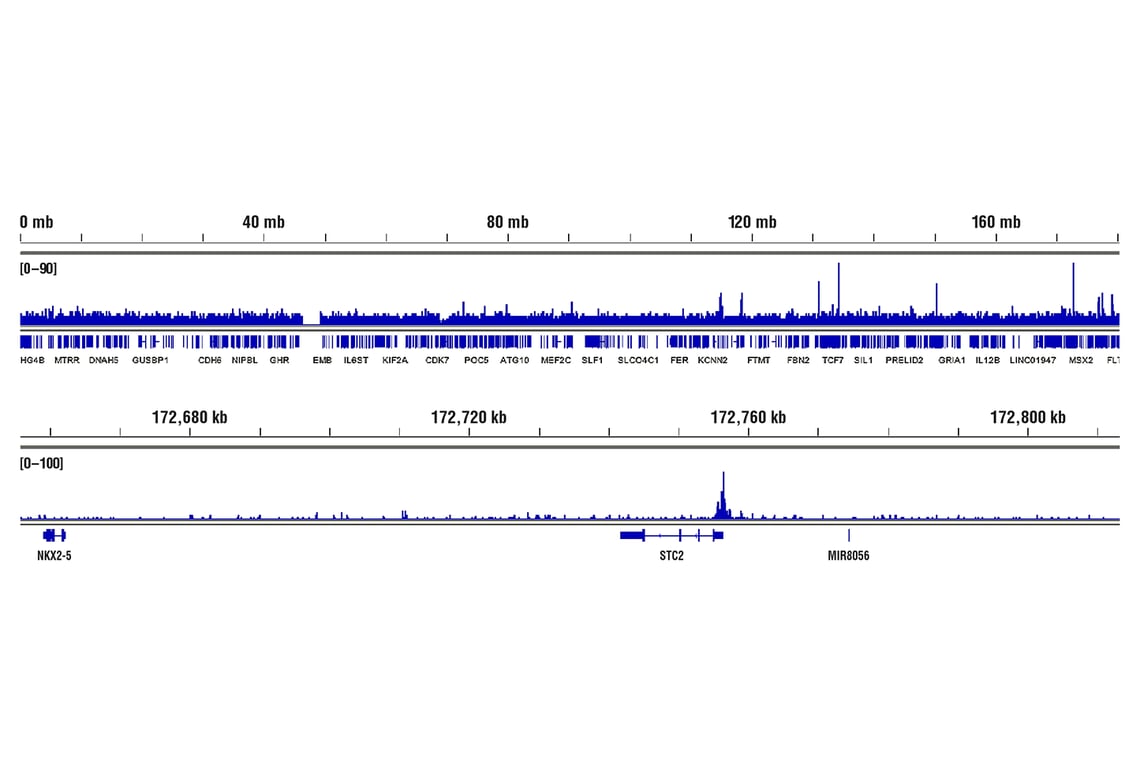
CUT&RUN was performed with MCF7 cells treated with cobalt chloride (100 μM) overnight and HIF-1α (D1S7W) XP® Rabbit mAb, using CUT&RUN Assay Kit #86652. DNA Libraries were prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figures show binding across chromosome 5 (upper), including STC2 (lower), a known target gene of HIF-1α (see additional figure containing CUT&RUN-qPCR data).
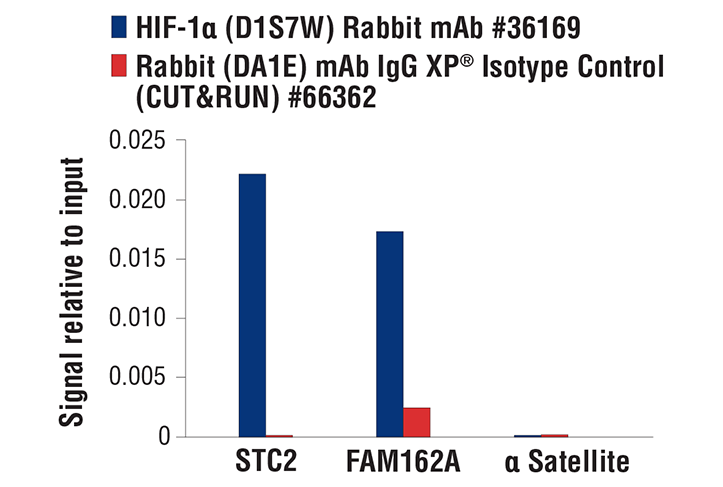
CUT&RUN was performed with MCF7 cells treated with cobalt chloride (100 μM) overnight and HIF-1α (D1S7W) XP® Rabbit mAb or Rabbit (DA1E) mAb IgG XP® Isotype Control (CUT&RUN) #66362, using CUT&RUN Assay Kit #86652. The enriched DNA was quantified by real-time PCR using human STC2 exon 1 primers, human FAM162A promoter primers and SimpleChIP® Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.