首页 抗体 一抗 内参抗体 BD Pharmingen™ Purified Mouse Anti-Human PARP
产品介绍
产品信息
简单描述
PARP [Poly(ADP-ribose) polymerase] is a 113 kDa nuclear chromatin-associated enzyme that catalyzes the transfer of ADP-ribose units from NAD+ to a variety of nuclear proteins including topoisomerases, histones, and PARP itself. The catalytic activity of PARP is increased in non-apoptotic cells following DNA damage, and PARP is thought to play an important role in mediating the normal cellular response to DNA damage. Additionally, PARP is a target of the caspase protease activity associated with apoptosis. During apoptosis, PARP is cleaved from a 113 kDa intact form into smaller 89 kDa and 24 kDa fragments. This process separates the amino-terminal DNA-binding domain of the enzyme from the carboxy-terminal catalytic domain resulting in the loss of normal PARP function. Although the role of PARP in apoptosis remains to be elucidated, PARP cleavage is considered to be a marker of apoptosis. The 4C10-5 antibody recognizes both the intact 113 kDa form and 89 kDa fragment of PARP.
The 4C10-5 antibody has been reported to recognize both native and denatured PARP. Purified human PARP was used as the immunogen and the antibody reported to react with an epitope located in the NAD binding domain. In dot blot assays, the antibody reacts with the native enzyme in the presence or absence of bound DNA as well as after synthesis of covalently linked poly (ADP-ribose). The 4C10-5 antibody is routinely tested by western blot analysis of untreated Jurkat T cells and Jurkat T cells induced to undergo apoptosis.

商品描述
4C10-5
PARP [Poly(ADP-ribose) polymerase] is a 113 kDa nuclear chromatin-associated enzyme that catalyzes the transfer of ADP-ribose units from NAD+ to a variety of nuclear proteins including topoisomerases, histones, and PARP itself. The catalytic activity of PARP is increased in non-apoptotic cells following DNA damage, and PARP is thought to play an important role in mediating the normal cellular response to DNA damage. Additionally, PARP is a target of the caspase protease activity associated with apoptosis. During apoptosis, PARP is cleaved from a 113 kDa intact form into smaller 89 kDa and 24 kDa fragments. This process separates the amino-terminal DNA-binding domain of the enzyme from the carboxy-terminal catalytic domain resulting in the loss of normal PARP function. Although the role of PARP in apoptosis remains to be elucidated, PARP cleavage is considered to be a marker of apoptosis. The 4C10-5 antibody recognizes both the intact 113 kDa form and 89 kDa fragment of PARP.
The 4C10-5 antibody has been reported to recognize both native and denatured PARP. Purified human PARP was used as the immunogen and the antibody reported to react with an epitope located in the NAD binding domain. In dot blot assays, the antibody reacts with the native enzyme in the presence or absence of bound DNA as well as after synthesis of covalently linked poly (ADP-ribose). The 4C10-5 antibody is routinely tested by western blot analysis of untreated Jurkat T cells and Jurkat T cells induced to undergo apoptosis.

产品详情
Purified
Tissue culture supernatant is purified by either protein A/G or affinity purification methods. Both methods yield antibody in solution that is free of most other soluble proteins, lipids, etc. This format provides pure antibody that is suitable for a number of downstream applications including: secondary labeling for flow cytometry or microscopy, ELISA, Western blot, etc.
应用
实验应用
Western blot (Routinely Tested), Bioimaging (Tested During Development), Dot Blot, Flow cytometry, Immunoprecipitation (Reported)
制备和贮存
存储溶液
Aqueous buffered solution containing ≤0.09% sodium azide.
保存方式
Aqueous buffered solution containing ≤0.09% sodium azide.
文献
文献
研发参考(2)
1. Patel T, Gores GJ, Kaufmann SH. The role of proteases during apoptosis. FASEB J. 1996; 10(5):587-597. (Biology).
2. Ranjit GB, Cheng MF, Mackay W, Whitacre CM, Berger JS, Berger NA. Poly(adenosine diphosphoribose) polymerase in peripheral blood leukocytes from normal donors and patients with malignancies. Clin Cancer Res. 1995; 1(2):223-234. (Clone-specific: Flow cytometry, Western blot).
参考图片
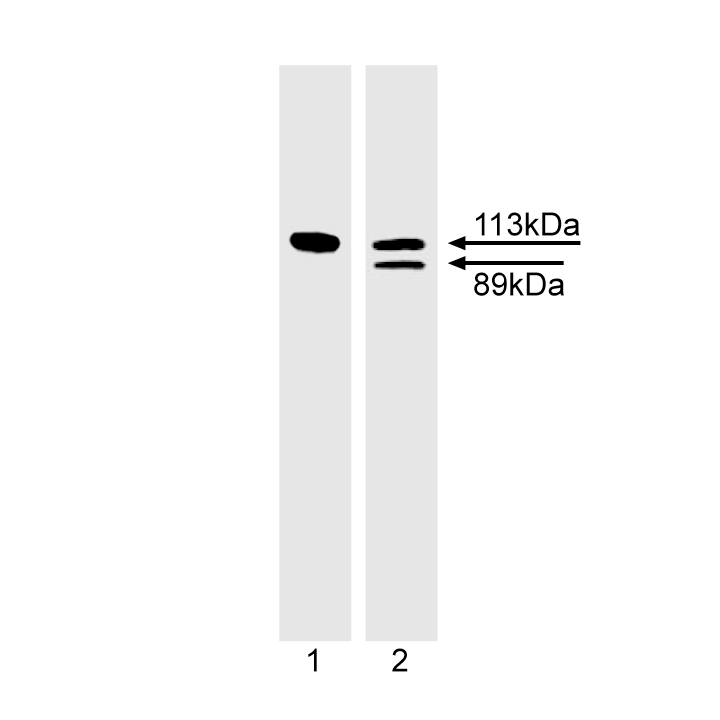
Western blot analysis of PARP cleavage. Jurkat cells were untreated (lane 1) or induced to undergo Fas mediated apoptosis by treatment with anti-human Fas mAb, clone DX2 (cat. No. 555670) and Protein G for 4 hr (lane 2) and probed with the PARP antibody at 1-2 µg/ml (clone 4C10-5). The 113 kDa intact form of PARP is seen in both the untreated and Fas mAb-treated cell lysates. However, the 89 kDa PARP cleavage fragment is only seen in the treated cell lysates.
Immunofluorescent staining of U-2 OS (ATCC HTB-96) cells. Cells were seeded in a 96 well imaging plate (Cat. No. 353219) at ~ 10 000 cells per well. After overnight incubation, cells were stained using the Perm Buffer III protocol and the anti-PARP antibody. The second step reagent was FITC goat anti mouse Ig (Cat. No. 554001). The image was taken on a BD Pathway™ 855 Bioimager using a 20x objective. This antibody also stained A549 (ATCC CCL-185) and HeLa (ATCC CCL-2) cells using both the Triton™ X-100 and Perm Buffer III protocols (see Recommended Assay Procedure).
当前规格1件起购
预计5-6个工作日送达,快递: 免运费,若需干冰额外收费
 全部商品分类
全部商品分类























 用小程序,查商品更便捷
用小程序,查商品更便捷




