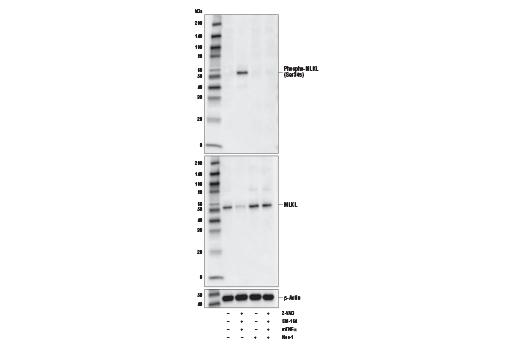

Necroptosis, a regulated pathway for necrotic cell death, is triggered by a number of inflammatory signals including cytokines in the tumor necrosis factor (TNF) family, pathogen sensors such as toll-like receptors (TLRs), and ischemic injury (1,2). The process is negatively regulated by caspases and is initiated through a complex containing the RIP1 and RIP3 kinases, typically referred to as the necrosome. Mixed lineage kinase domain-like protein (MLKL) is a pseudokinase that was identified as a downstream target of RIP3 in the necroptosis pathway (3,4). During necroptosis RIP3 is phosphorylated at Ser227, which recruits MLKL and leads to its phosphorylation at Thr357 and Ser358 (3). Knockdown of MLKL through multiple mechanisms results in inhibition of necroptosis (3-5). While the precise mechanism for MLKL-induced necroptosis is unclear, some studies have shown that necroptosis leads to oligomerization of MLKL and translocation to the plasma membrane, where it affects membrane integrity (6-9). 1.Christofferson, D.E. and Yuan, J. (2010) Curr Opin Cell Biol 22, 263-8. 2.Kaczmarek, A. et al. (2013) Immunity 38, 209-23. 3.Sun, L. et al. (2012) Cell 148, 213-27. 4.Wang, Z. et al. (2012) Cell 148, 228-43. 5.Wu, J. et al. (2013) Cell Res 23, 994-1006. 6.Cai, Z. et al. (2014) Nat Cell Biol 16, 55-65. 7.Chen, X. et al. (2014) Cell Res 24, 105-21. 8.Wang, H. et al. (2014) Mol Cell 54, 133-46. 9.Dondelinger, Y. et al. (2014) Cell Rep 7, 971-81.








 用小程序,查商品更便捷
用小程序,查商品更便捷







 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)