 全部商品分类
全部商品分类


Monoclonal antibody is produced by immunizing animals with a synthetic phosphopeptide corresponding to residues surrounding Ser2/Ser5 of the human Rpb1 CTD heptapeptide repeat.


Product Usage Information
For optimal ChIP and ChIP-seq results, use 10 μl of antibody and 10 μg of chromatin (approximately 4 x 106 cells) per IP. This antibody has been validated using SimpleChIP® Enzymatic Chromatin IP Kits.
The CUT&RUN dilution was determined using CUT&RUN Assay Kit #86652.
The CUT&Tag dilution was determined using CUT&Tag Assay Kit #77552.
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
| Simple Western™ | 1:50 - 1:250 |
| Immunoprecipitation | 1:50 |
| Chromatin IP | 1:50 |
| Chromatin IP-seq | 1:50 |
| CUT&RUN | 1:50 |
| CUT&Tag | 1:50 |



Specificity/Sensitivity
Species Reactivity:
Human, Mouse, Rat, Monkey




Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% sodium azide. Store at –20°C. Do not aliquot the antibody.


参考图片
Peptide dot blot analysis demonstrating Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb specificity. Antibody binding to pre-coated Rpb1 CTD peptides is shown using Phospho-Rpb1 CTD (Ser2) (E1Z3G) Rabbit mAb #13499, Phospho-Rpb1 CTD (Ser5) (D9N5I) Rabbit mAb #13523, a phospho-Rpb1 CTD (Ser7) antibody, and Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb. As expected, Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb only binds to phospho-Rpb1 CTD peptide when phosphorylated at both Ser2 and Ser5.
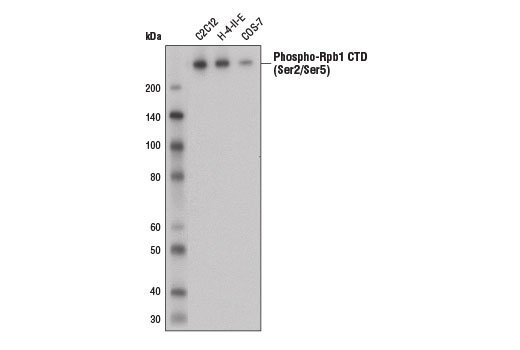
Western blot analysis of extracts from C2C12, H-4-II-E, and COS-7 cells using Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb.
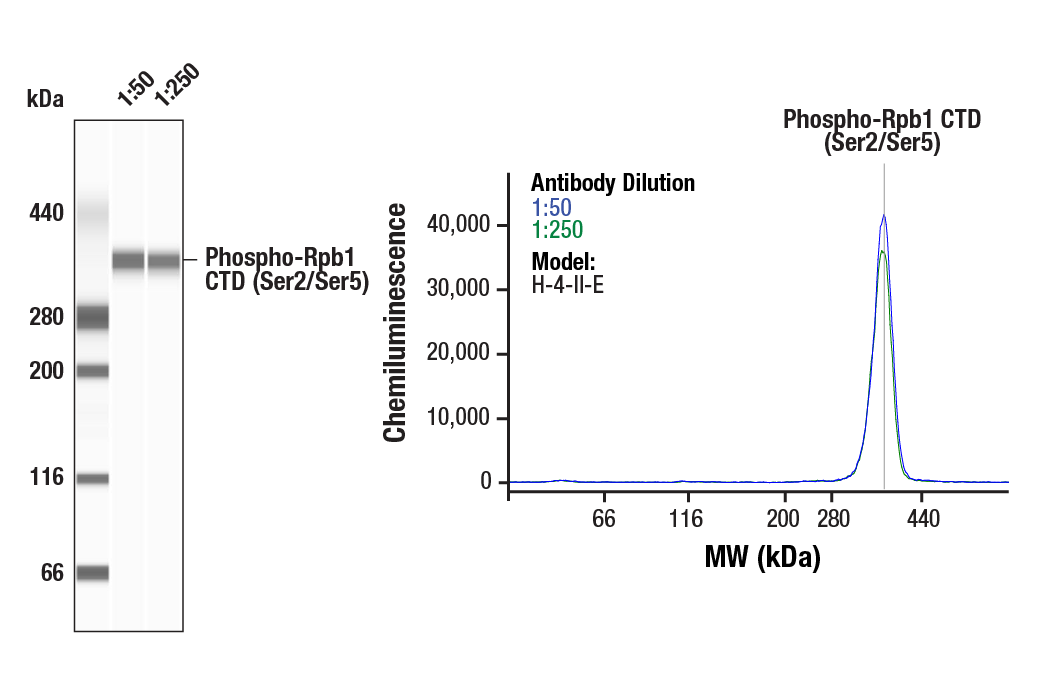
Simple Western™ analysis of lysates (0.1 mg/mL) from H-4-II-E cells using Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb #13546. The virtual lane view (left) shows the target band (as indicated) at 1:50 and 1:250 dilutions of primary antibody. The corresponding electropherogram view (right) plots chemiluminescence by molecular weight along the capillary at 1:50 (blue line) and 1:250 (green line) dilutions of primary antibody. This experiment was performed under reducing conditions on the Jess™ Simple Western instrument from ProteinSimple, a BioTechne brand, using the 66-440 kDa separation module.
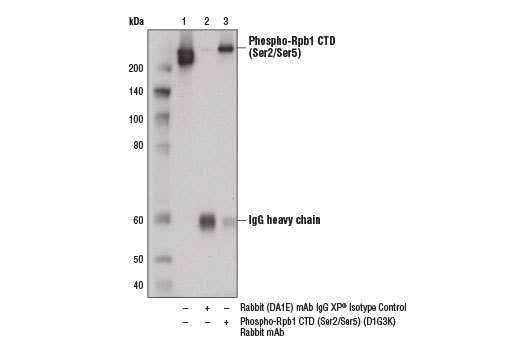
Immunoprecipitation of Rpb1 from HeLa cell extracts using Rabbit (DA1E) mAb IgG XP® Isotype Control #3900 (lane 2) or Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb (lane 3). Lane 1 is 10% input. Western blot analysis was performed using Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb.
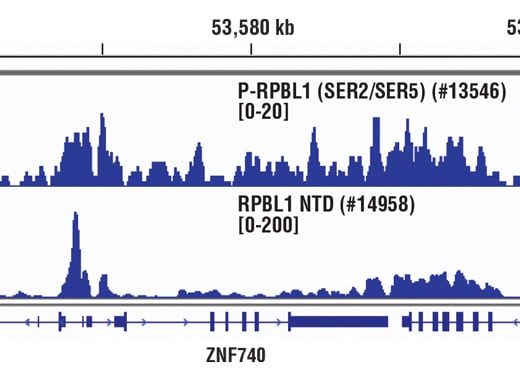
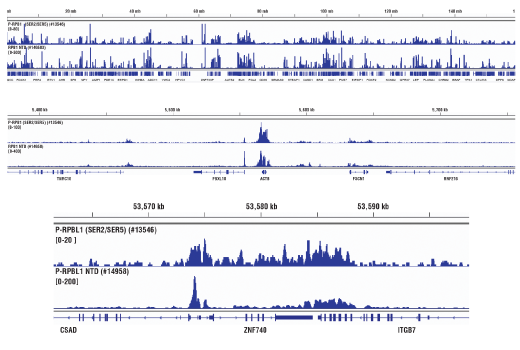
Chromatin immunoprecipitations were performed with cross-linked chromatin from Hela cells and either Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb or Rpb1 NTD (D8L4Y) Rabbit mAb #14958, using SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) #9003. DNA Libraries were prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figure shows binding across the ZNF740 gene on chromosome 12. For additional ChIP-seq tracks, please download the product datasheet.
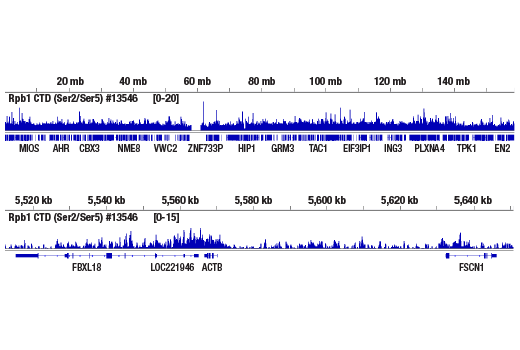
Chromatin immunoprecipitations were performed with cross-linked chromatin from Hela cells and either Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb or of Rpb1 NTD (D8L4Y) Rabbit mAb #14958, using SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) #9003. DNA Libraries were prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figure shows binding across chromosome 7 (upper), including ACTB (medium), a known target gene of Phospho-Rpb1 CTD (Ser2/Ser5) (see additional figure containing ChIP-qPCR data), and ZNF740 gene on chromosome 12 (lower).
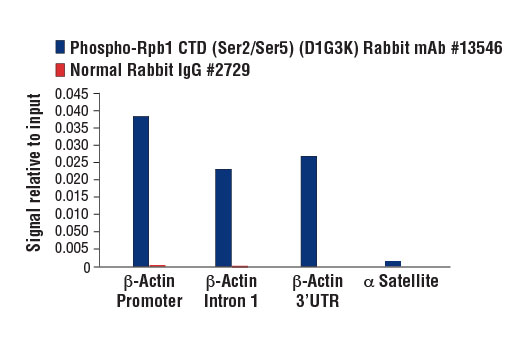
Chromatin immunoprecipitations were performed with cross-linked chromatin from HeLa cells and either Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb or Normal Rabbit IgG #2729 using SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) #9003. The enriched DNA was quantified by real-time PCR using SimpleChIP® Human β-Actin Promoter Primers #13653, human Β-Actin intron 1 primers, SimpleChIP® Human β-Actin 3' UTR Primers #13669, and SimpleChIP® Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.
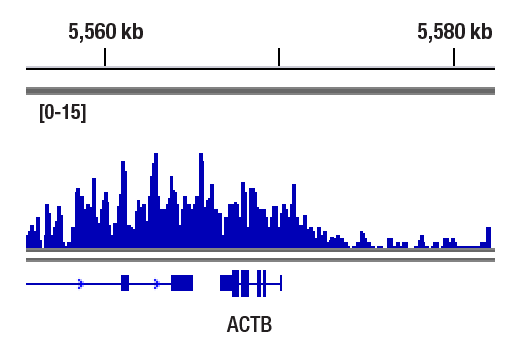
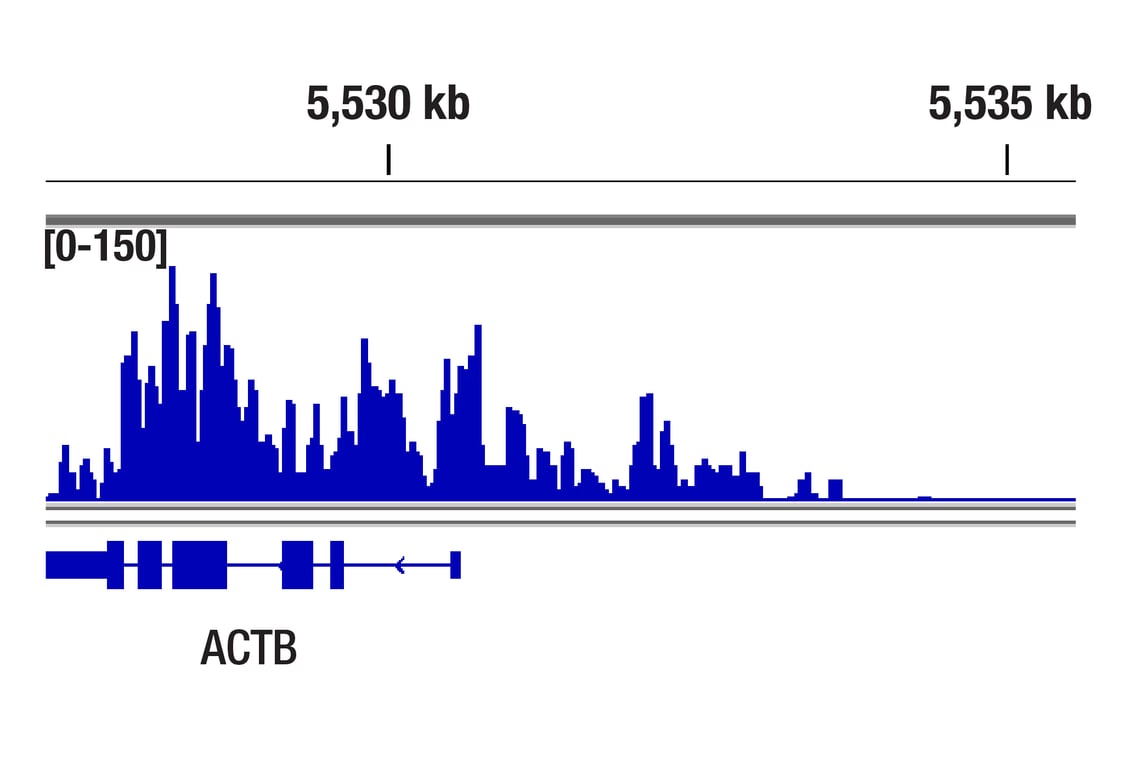
CUT&RUN was performed with HeLa cells and Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb, using CUT&RUN Assay Kit #86652. DNA Library was prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figure shows binding across ACTB gene.
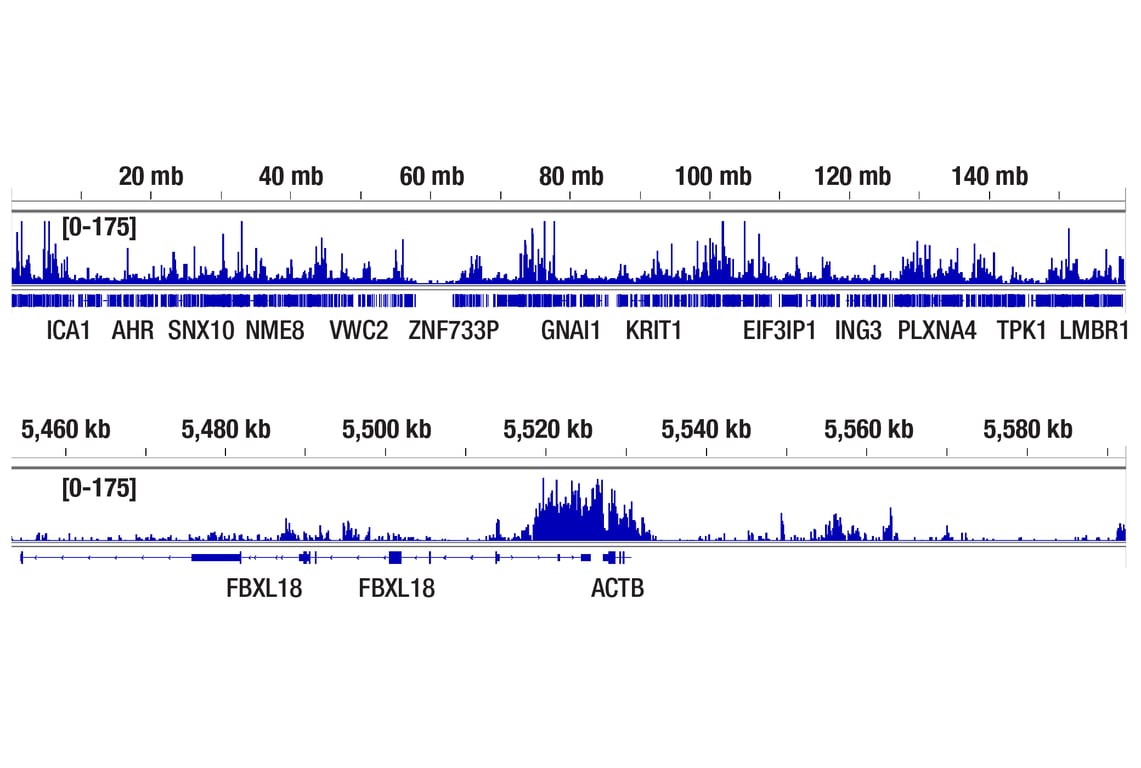
CUT&RUN was performed with HeLa cells and Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb, using CUT&RUN Assay Kit #86652. DNA Library was prepared using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795. The figures show binding across chromosome 7 (upper), including ACTB gene (lower).
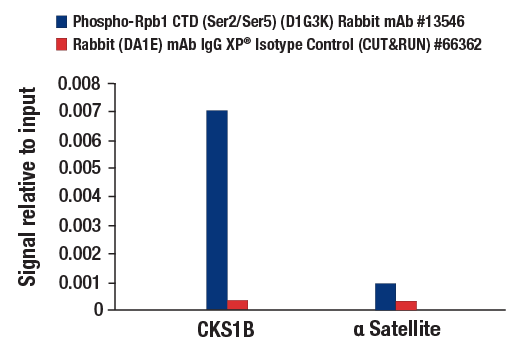
CUT&RUN was performed with HeLa cells and either Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb or Rabbit (DA1E) mAb IgG XP® Isotype Control (CUT&RUN) #66362, using CUT&RUN Assay Kit #86652. The enriched DNA was quantified by real-time PCR using human CKS1B exon 1 primers and SimpleChIP® Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.
CUT&Tag was performed with HeLa cells and Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb, using CUT&Tag Assay Kit #77552. DNA library was prepared using CUT&Tag Dual Index Primers and PCR Master Mix for Illumina Systems #47415. The figure shows binding across ACTB, a known target gene of Rpb1 (see our ChIP-qPCR figure).
CUT&Tag was performed with HeLa cells and Phospho-Rpb1 CTD (Ser2/Ser5) (D1G3K) Rabbit mAb, using CUT&Tag Assay Kit #77552. DNA library was prepared using CUT&Tag Dual Index Primers and PCR Master Mix for Illumina Systems #47415. The figures show binding across chromosome 7 (upper), including ACTB (lower), a known target gene of Rpb1 (see our ChIP-qPCR figure).





 用小程序,查商品更便捷
用小程序,查商品更便捷




