 全部商品分类
全部商品分类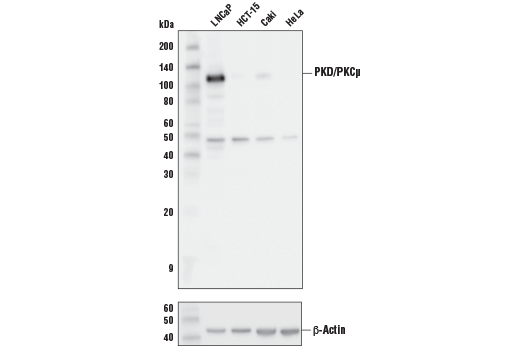

Activation of PKC is one of the earliest events in a cascade leading to a variety of cellular responses, such as secretion, gene expression, proliferation, and muscle contraction (1,2). Protein kinase D (PKD), also called PKCμ, is a serine/threonine kinase whose activation is dependent on the phosphorylation of two activation loop sites, Ser744 and Ser748, via a PKC-dependent signaling pathway (3-5). In addition to the two activation loop sites, the carboxy-terminal Ser916 has been identified as an autophosphorylation site for PKD/PKCμ. Phosphorylation at Ser916 correlates with PKD/PKCμ catalytic activity (6). 1.Nishizuka, Y. (1984) Nature 308, 693-698. 2.Keranen, L.M. (1995) Curr. Biol. 5, 1394-1403. 3.Valverde, A.M. et al. (1994) Proc. Natl. Acad. Sci. 91, 8572-8576. 4.Johannes, F.J. et al. (1994) J. Biol. Chem. 269, 6140-6148. 5.Iglesias, T. et al. (1998) J. Biol. Chem. 273, 27662-27667. 6.Matthews, S.A. et al. (1999) J. Biol. Chem. 274, 26543-26549.








 用小程序,查商品更便捷
用小程序,查商品更便捷





