 全部商品分类
全部商品分类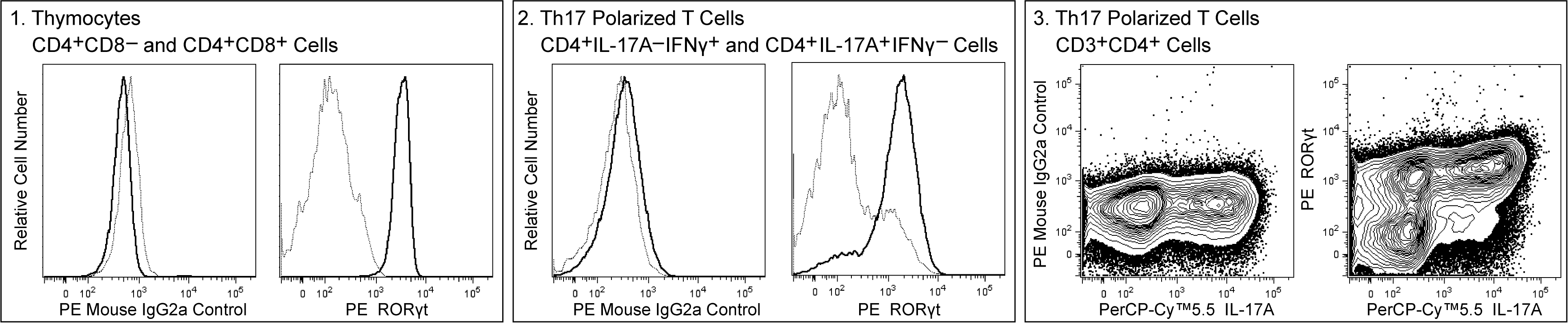










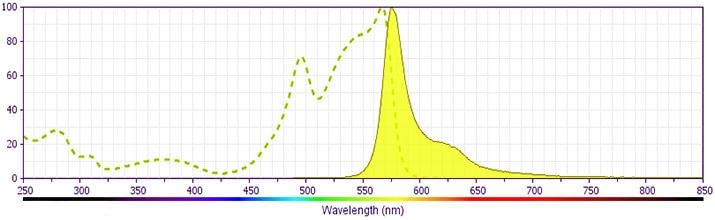






参考图片
Multicolor flow cytometric analysis of mouse RORγt expression in thymocytes and Th17 polarized cells. BALB/c mouse thymocytes (Panel 1) or Th17 polarized cells (Panels 2 and 3) were stained with BD Horizon™ Fixable Viability Stain 450 (Cat. No. 562247), fixed and permeabilized using the BD Pharmingen™ Transcription Factor Buffer Set (Cat. No. 562574) and then stained with either PE Mouse Anti-Mouse RORγt antibody (Cat. No. 562607) or PE Mouse IgG2a, κ Isotype Control (Cat. No. 554648). The thymocytes were counterstained with APC Rat Anti-Mouse CD4 (Cat. No. 553051) and PerCP-Cy™5.5 Rat Anti-Mouse CD8a (Cat. No. 551162) antibodies. The Th17-polarized cells were counterstained with APC-Cy™7 Hamster Anti-Mouse CD3e (Cat. No. 557596), Alexa Fluor® 700 Rat Anti-Mouse CD4 (Cat. No. 557956), FITC Rat Anti-Mouse IFN-γ (Cat. No. 554411) and PerCP-Cy™5.5 Rat Anti-Mouse IL-17A (Cat. No. 560666) antibodies. Panel 1: The overlapping histograms show the levels of IgG2a Control (Left) and RORγt (Right) staining in CD4+CD8- single-positive (dotted line) or CD4+CD8+ double-positive (solid line) thymocytes. Panel 2: The overlapping histograms show the levels of IgG2a Control (Left) and RORγt (Right) staining in Th17-polarized IL-17A-IFN-γ+ (dotted line) or IL-17A+IFN-γ- (solid line) cells. Panel 3: Two-color contour plots show the correlated expression patterns of IgG2a Control (Left) or ROR γ t (Right) staining versus IL-17A in Th17-polarized CD3+CD4+ cells. The flow cytometric data were derived from viable cell-discriminated events with the forward and side light-scatter characteristics of intact single cells using a BD LSRFortessa™ Cytometer System.






 用小程序,查商品更便捷
用小程序,查商品更便捷




