 全部商品分类
全部商品分类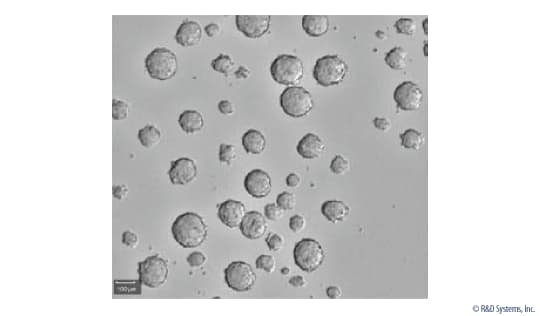

 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷


 收藏
收藏
 对比
对比 咨询
咨询Media Components
- 100 mL of StemXVivo® Serum-Free Tumorsphere Media.

Scientific Data
 View Larger
View LargerTumorspheres Formed from the MCF-7 Human Breast Cancer Cell Line. MCF-7 human breast cancer cells were suspended in StemXVivo®Serum-Free Tumorsphere Media (Catalog # CCM012) containing 2 U/mL Heparin (Tocris Catalog # 2812) and 0.8 µg/mL Hydrocortisone (Tocris, Catalog # 4093). The cells were plated at 3 x 104cells per well in a 6-well ultralow adhesion culture plate and cultured at 37°C and 5% CO2for 3-10 days to induce tumorsphere formation. Tumorspheres were imaged using a CloneSelect™Imager from Molecular Devices (New Milton) Limited.
Assay Procedure
Refer to the product datasheet for complete product details.
Briefly, tumorsphere formation can be induced using the following procedure
- Cells are suspended in StemXVivo Serum-Free Tumorsphere Media containing Heparin and Hydrocortisone
- Cells are cultured on low adhesion plates at 37 °C and 5% CO2
- Tumorspheres should form in 3-10 days
Reagents supplied in the StemXVivo® Serum-Free Tumorsphere Media (Catalog # CCM012):
- 100 mL of StemXVivo® Serum-Free Tumorsphere Media
Reagents
Materials
- 15 mL conical tube
- Ultralow adhesion cell culture plates
Equipment
- 37 °C and 5% CO2 incubator
- Benchtop Centrifuge
Cryopreservation of Stem Cells
Add 2 U/mL Heparin and 0.5 µ;g/mL Hydrocortisone to pre-warmed StemXVivo® Serum-Free Tumorsphere Media.

Resuspend cells in the supplemented media.

Transfer the cell suspension to ultralow adhesion plates.

Incubate cultures in a 5% CO2 and 37 °C incubator.
Tumorspheres should form in 3-10 days.

StemXVivo Serum-Free Tumorsphere Media Summary
Semi-solid media formulated and optimized for tumorsphere formation.
Key Benefits
- Shown to support sphere formation of seven tumor cell lines
- Lot-to-lot consistency reduces experimental variability
- Specially formulated and optimized for tumorsphere formation
Why Perform the Tumorsphere Assay Using Specially Formulated Media?
Tumorsphere assays, also called sphere-formation assays, are in vitro assays that assess stem cell characteristics of tumor cells. There are a number of variables that contribute to variation in data obtained from tumorsphere assays including the purity of the starting cell population, the type and quantity of media and supplements, and the potential for tumorspheres to aggregate.
To minimize experimental variability due to the media and supplements, R&D Systems offers StemXVivo® Serum-Free Tumorsphere Media, which is formulated and optimized for tumorsphere formation.
StemXVivo® Serum-Free Tumorsphere Media:
- Shown to support sphere formation of seven tumor cell lines.
- Formulated and optimized for efficient tumorsphere formation.
- Lot-to-lot consistency reduces experimental variability.
Media Components
- 100 mL of StemXVivo® Serum-Free Tumorsphere Media.
Stability and Storage
Upon receipt, StemXVivo® Serum-Free Tumorsphere Media should be stored at =-20 °C in a manual defrost freezer. The media can be thawed at 2 °C to 8 °C or at room temperature. Thawed media can be aliquoted and stored at =-20°C in a manual defrost freezer for up to 3 months or used within two weeks when stored in the dark at 2 °C to 8 °C. Avoid repeated freeze-thaw cycles. Do not use this product beyond the expiration date.
Precautions
This product contains human transferrin. The transferrin was tested at the donor level using an FDA licensed method and found to be non-reactive for anti-HIV-1/2 and Hepatitis B surface antigen. As no testing can offer complete assurance of freedom from infectious agents, this product should be handled as if capable of transmitting infection.
Limitations
- FOR LABORATORY RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES.
- The safety and efficacy of this product in diagnostic or other clinical uses has not been established.
- This reagent should not be used beyond the expiration date indicated on the label.
- Results may vary with cells cultured by different methods.





