 全部商品分类
全部商品分类









参考图片
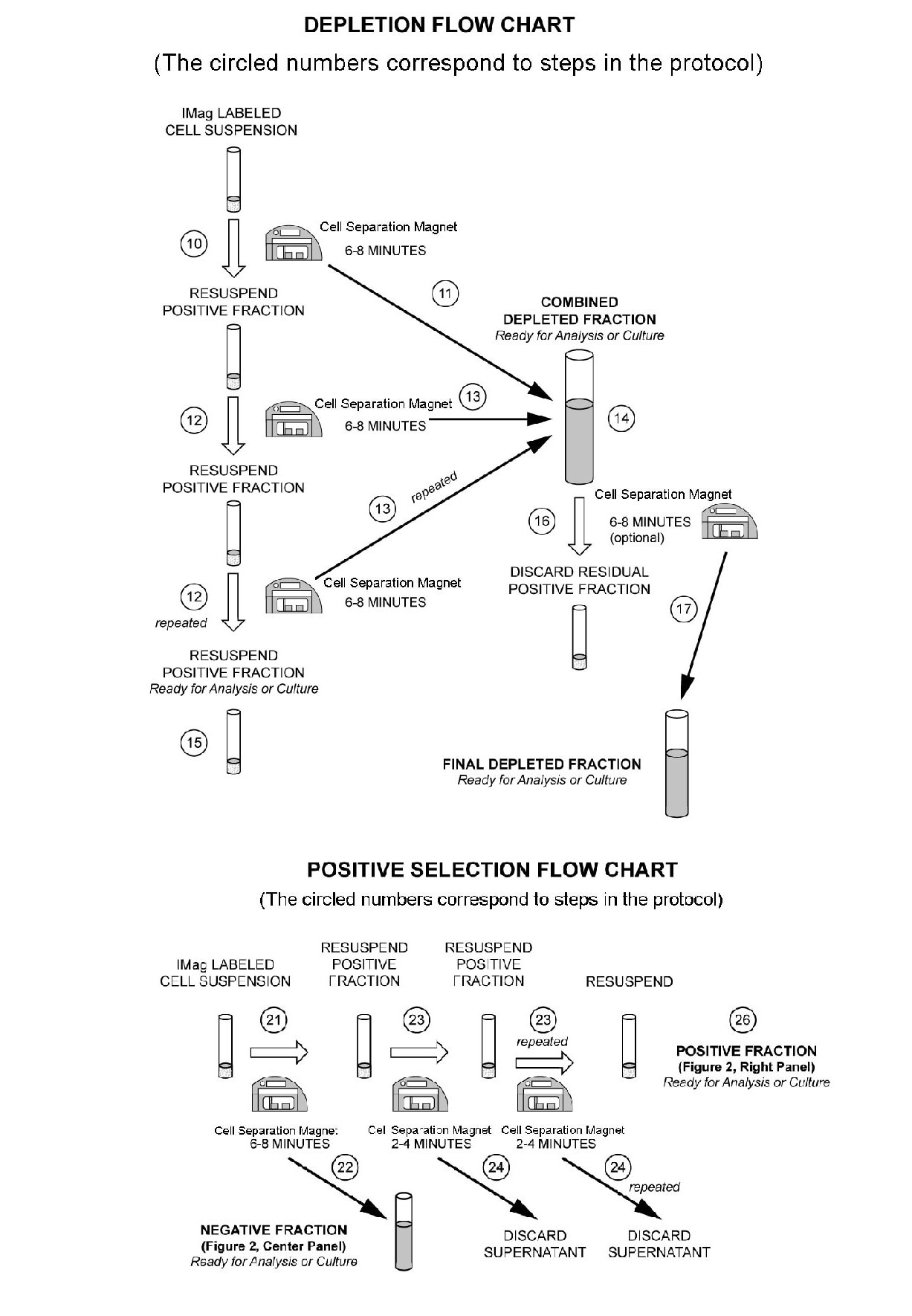
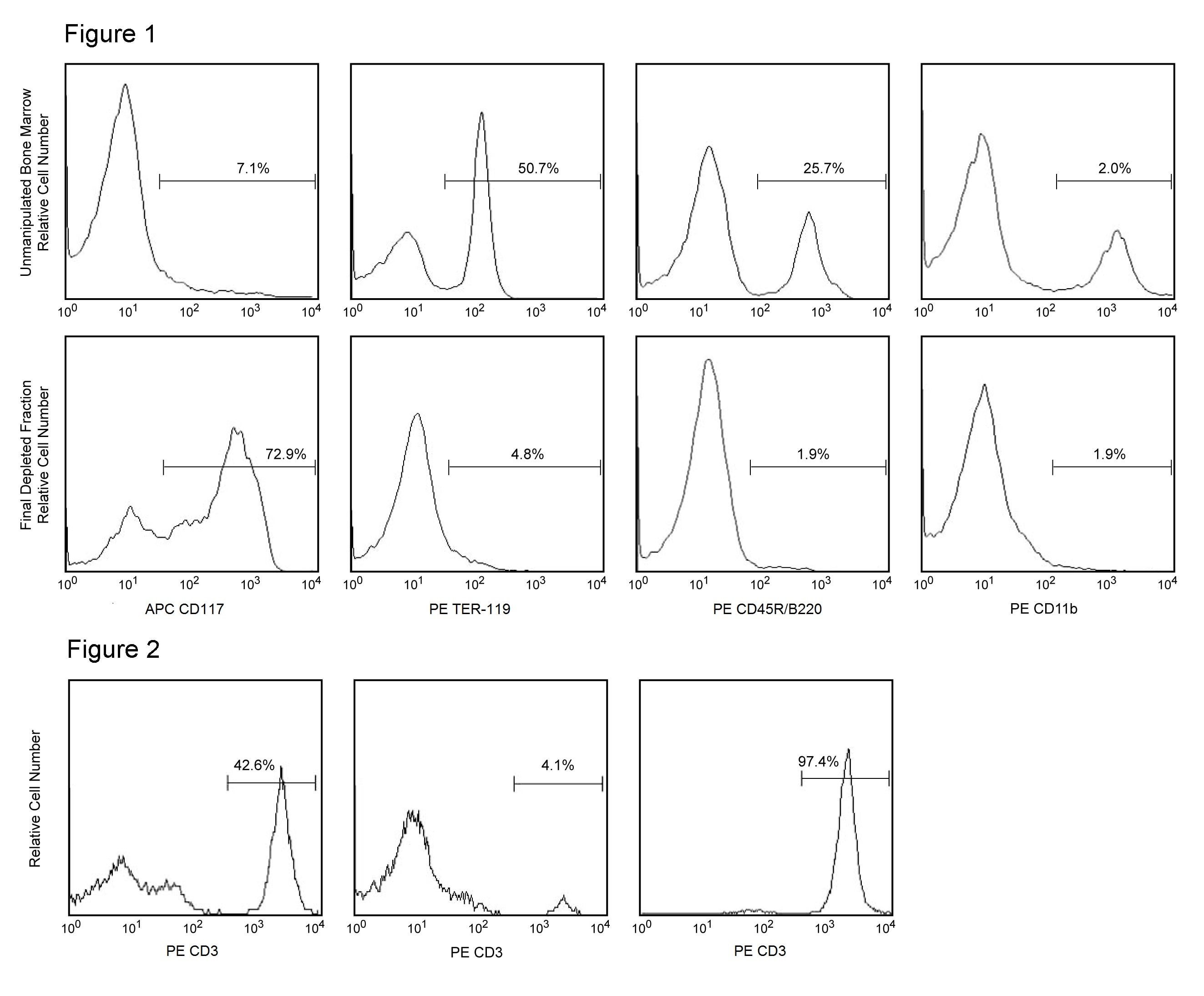
Figure 1. Depletion of lineage-committed cells from mouse bone marrow. BALB/c bone-marrow cells were labeled with BD™ IMag Mouse Hematopoietic Progenitor Enrichment Set - DM (Cat. No. 558451) containing Biotin Anti-Mouse CD3, Anti-Mouse CD11b, Anti-Mouse CD45R/B220, Anti-Mouse Ly-6G and Ly-6C, and Anti-Mouse Erythroid Cells mAbs. After washing, the cells were labeled with BD™ IMag Streptavidin Particles Plus - DM (Cat. No. 557812) at 50 µl/1x10^7 total cells. The labeled cells were then separated on a BD™ IMag Cell Separation Magnet (Cat. No. 552311). Unmanipulated bone marrow cells and the final depleted fraction were stained with APC Anti-Mouse CD117 (Cat. No. 553356) to detect hematopoietic progenitors, and with PE Anti-Mouse TER-119 (Cat. No. 553673), CD45R/B220 (Cat. No. 553089/553090), and CD11b (Cat. No. 557397/553311) mAbs to detect lineage-commited cells. The percentage of positive cells is indicated in each panel; placement of each marker is based upon staining with the appropriate isotype control (data not shown). The final depleted fraction contains an increased proportion of CD117+ cells and less than 5% of lineage-positive contaminants. Figure 2. Positive selection of human CD3-positive lymphocytes. Peripheral blood mononuclear cells (PBMC) were labeled with Biotin Anti-Human CD3 mAb (Cat. No. 555338) and BD™ IMag Streptavidin Particles - DM at 20 µl/10^7 total cells. After labeling, the cells were separated using the Cell Separation Magnet, and the negative (CD3 ) and positive (CD3+) fractions were collected. Please refer to the Positive Selection Flow Chart to identify the separated cell populations represented in this figure. For flow cytometric analysis, fresh PBMC (left panel), the negative fraction (middle panel), and the positive fraction (right panel) were stained with PE Anti-human CD3 mAb (Cat. No. 555333). The percent CD3+ cells in each sample is given. Flow cytometry was performed on a BD FACSCalibur™ flow cytometry system.
Figure 1. Depletion of lineage-committed cells from mouse bone marrow. BALB/c bone-marrow cells were labeled with BD™ IMag Mouse Hematopoietic Progenitor Enrichment Set - DM (Cat. No. 558451) containing Biotin Anti-Mouse CD3, Anti-Mouse CD11b, Anti-Mouse CD45R/B220, Anti-Mouse Ly-6G and Ly-6C, and Anti-Mouse Erythroid Cells mAbs. After washing, the cells were labeled with BD™ IMag Streptavidin Particles Plus - DM (Cat. No. 557812) at 50 µl/1x10^7 total cells. The labeled cells were then separated on a BD™ IMag Cell Separation Magnet (Cat. No. 552311). Unmanipulated bone marrow cells and the final depleted fraction were stained with APC Anti-Mouse CD117 (Cat. No. 553356) to detect hematopoietic progenitors, and with PE Anti-Mouse TER-119 (Cat. No. 553673), CD45R/B220 (Cat. No. 553089/553090), and CD11b (Cat. No. 557397/553311) mAbs to detect lineage-commited cells. The percentage of positive cells is indicated in each panel; placement of each marker is based upon staining with the appropriate isotype control (data not shown). The final depleted fraction contains an increased proportion of CD117+ cells and less than 5% of lineage-positive contaminants. Figure 2. Positive selection of human CD3-positive lymphocytes. Peripheral blood mononuclear cells (PBMC) were labeled with Biotin Anti-Human CD3 mAb (Cat. No. 555338) and BD™ IMag Streptavidin Particles - DM at 20 µl/10^7 total cells. After labeling, the cells were separated using the Cell Separation Magnet, and the negative (CD3 ) and positive (CD3+) fractions were collected. Please refer to the Positive Selection Flow Chart to identify the separated cell populations represented in this figure. For flow cytometric analysis, fresh PBMC (left panel), the negative fraction (middle panel), and the positive fraction (right panel) were stained with PE Anti-human CD3 mAb (Cat. No. 555333). The percent CD3+ cells in each sample is given. Flow cytometry was performed on a BD FACSCalibur™ flow cytometry system.






 用小程序,查商品更便捷
用小程序,查商品更便捷




