 全部商品分类
全部商品分类

 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷


 收藏
收藏
 对比
对比 咨询
咨询Scientific Data
 View Larger
View LargerCarrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
645-WN
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS, EDTA and CHAPS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
645-WN/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS, EDTA and CHAPS. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
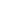
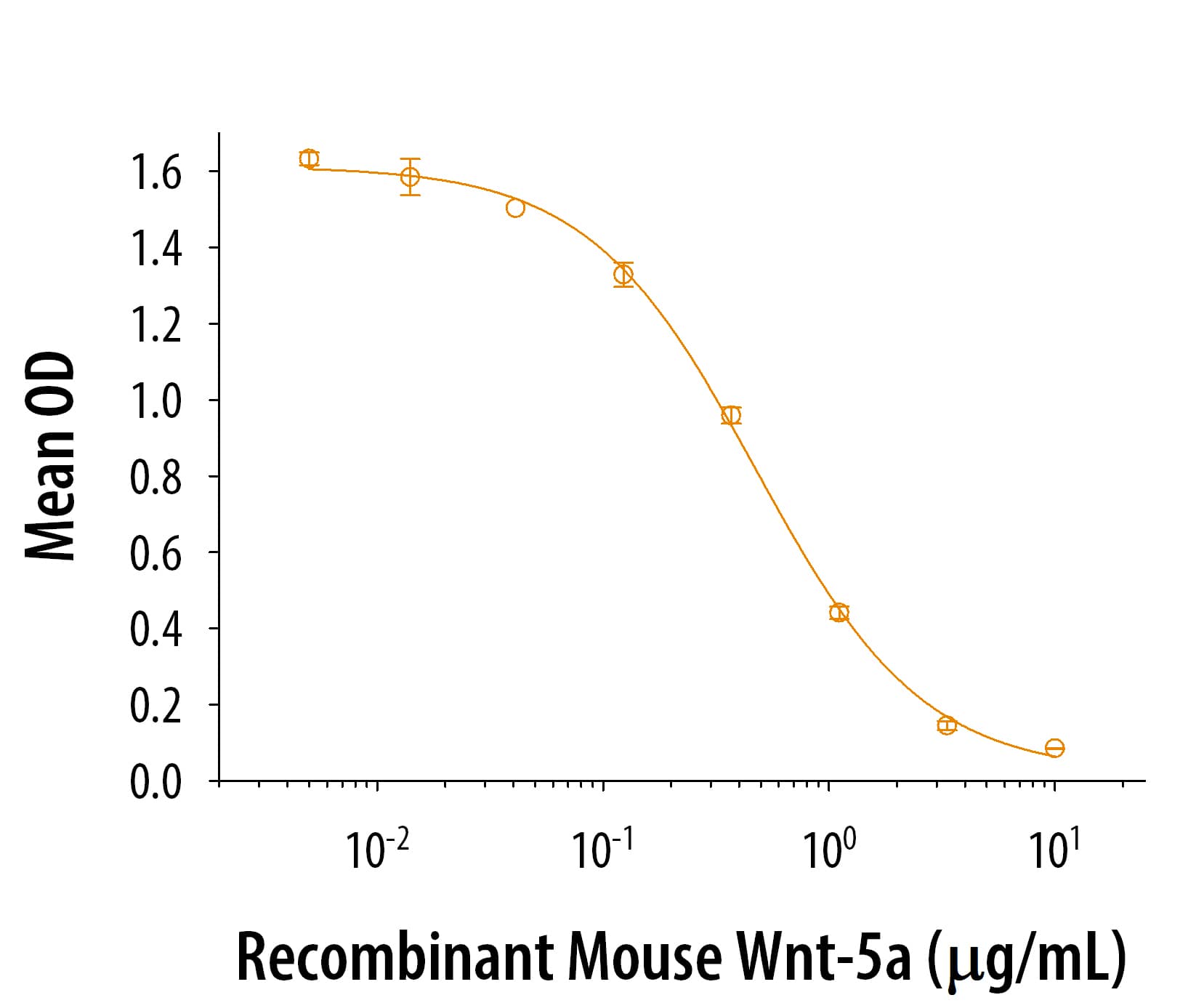
Recombinant Human/Mouse Wnt-5a Protein Summary
Product Specifications
Optimal concentrations should be determined by each laboratory for each application.
Gln38-Lys380
Analysis
Background: Wnt-5a
Wnt-5a is a 44‑50 kDa member of the Wnt family of proteins (1‑6). Based on its activity towards C57Mg mammary epithelium, it is classified as a nontransforming Wnt. Human Wnt‑5a is synthesized as a 380 amino acid (aa) precursor that contains a 37 aa signal sequence, a 25 aa prosegment, and a 319 aa mature region (1, 2, 3). The mature region has 24 cysteine residues that form multiple intrachain disulfide bonds, plus four N‑linked glycosylation sites that are utilized for proper secretion (3, 5, 7). There is also a palmitate adduct at Cys104 that is essential for activity, and a potential palmitoleic acid modification at Ser244 that may also contribute to secretion (7‑9). One alternative start site is reported at Met16. Over aa 38‑380, human and mouse Wnt‑5a are identical in amino acid sequence (1, 10). Cells known to express Wnt‑5a include brainstem astrocytes (11), mammary epithelium (12), CD34+ primitive progenitor stem cells (13), chondrocytes (14), CD34- pericytes and vascular smooth muscle cells (15), plus mesenchymal cells at various sites (16, 17). There are multiple receptors for Wnt‑5a. These include Fzd-1, -2,
-3, -4, -5, and -7 (3, 18‑22), Ror2 (3), LRP6 (23), Ryk (24) and sFRP1 (25). All these molecules function within the context of a larger number of “co‑factors” that regulate signaling by the Wnts. Initially, it was suggested that there were three pathways for Wnt signaling; a beta -catenin-mediated canonical pathway, and two noncanonical pathways described as the Wnt/JNK (PCP) pathway and the Wnt/Ca++ pathway (26, 27). And it was assumed that various Wnts could be accommodated by these classifications. At present, it is now recognized that individual Wnts, through various combinations of receptor complex subunits, can have diverse effects, perhaps even within the same cell (3, 6, 27). Further complexity is introduced by the fact that Xenopus Wnt‑5a and Wnt‑11 are known to form bioactive heterodimers following Tyr sulfation (28). Thus, predicting the activity of Wnt‑5a, or any other Wnt, on any cell type will require substantial insight into the interaction between all the extracellular, cell surface and intracellular components of the Wnt signaling system.
- Clark, C.C. et al. (1993) Genomics 18:249.
- LeJeune, S. et al. (1995) Clin. Cancer Res. 1:215.
- Mikels, A.J. & R. Nusse (2006) PLoS Biol. 4:e115.
- Nishita, M. et al. (2010) Trends Cell Biol. 20:346.
- Mikels, A.J. & R. Nusse (2006) Oncogene 25:7461.
- van Amerongen, R. & R. Nusse (2009) Development 136:3205.
- Kurayoshi, M. et al. (2007) Biochem. J. 402:515.
- Takada, R. et al. (2006) Dev. Cell 11:791.
- Port, F. & K. Basler (2010) Traffic May 3. [Epub ahead of print].
- Gavin, B.J. et al. (1990) Genes Dev. 4:2319.
- Castelo-Branco, G. et al. (2006) Mol. Cell. Neurosci. 31:251.
- Jonsson, M. et al. (1998) Br. J. Cancer 78:430.
- van Den Berg, D.J. et al. (1998) Blood 92:3189.
- Kruger, C. & C. Kappen (2010) PLoS One 5:e8978.
- Lin, G. et al. (2008) Stem Cells Dev. 17:1053.
- Lickert, H. et al. (2001) Mech. Dev. 105:181.
- Danielson, K.G. et al. (1995) J. Biol. Chem. 270:31225.
- Gazit, A. et al. (1999) Oncogene 18:5959.
- Bazhin, A. V. et al. (2010) Cell. Mol. Life Sci. 67:817.
- Kawasaki, A. et al. (2007) Cell. Signal. 19:2498.
- Blumenthal, A. et al. (2006) Blood 108:965.
- Umbhauer, M. et al. (2000) EMBO J. 19:4944.
- Bryja, V. et al. (2009) Mol. Biol. Cell 20:924.
- Keeble, T.R. et al. (2006) J. Neurosci. 26:5840.
- Lin, K. et al. (1997) Proc. Natl. Acad. Sci. USA 94:11196.
- Rao, T.P. & M. Kuhl (2010) Circ. Res. 106:1798.
- McDonald, S.L. & A. Silver (2009) Br. J. Cancer 101:209.
- Cha, S-W. et al. (2009) Curr. Biol. 19:1573.






