全部商品分类
全部商品分类



 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷


 收藏
收藏
 对比
对比 咨询
咨询Immunohistochemistry(5-15 µg/mL)



Gln254-Cys334
Accession # P22725

Scientific Data
 View Larger
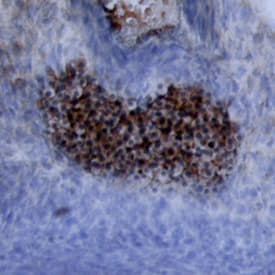
View LargerWnt‑5a in Mouse Embryonic Rib. Wnt‑5a was detected in immersion fixed paraffin-embedded sections of mouse embryonic rib using 15 µg/mL Mouse/Rat Wnt‑5a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF645) overnight at 4 °C. Tissue was stained with the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View LargerWnt‑5a in Mouse Embryo. Wnt-5a was detected in immersion fixed frozen sections of mouse embryo using Mouse/Rat Wnt-5a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF645) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
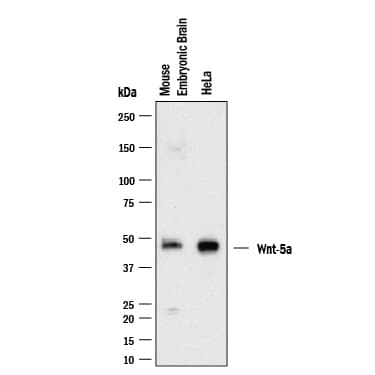
View LargerDetection of Mouse/Rat Wnt‑5a by Western Blot. Western blot shows lysates of HeLa Human Cervical Epithelial Carcinoma Cells and Mouse Brain (embryo E14). PVDF membrane was probed with 2 µg/mL of Goat Anti-Mouse/Rat Wnt‑5a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF645) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for Wnt‑5a at approximately ~42kDa kDa (as indicated). This experiment was conducted under reducing conditions and using Western Blot Buffer Group 1.
 View Larger
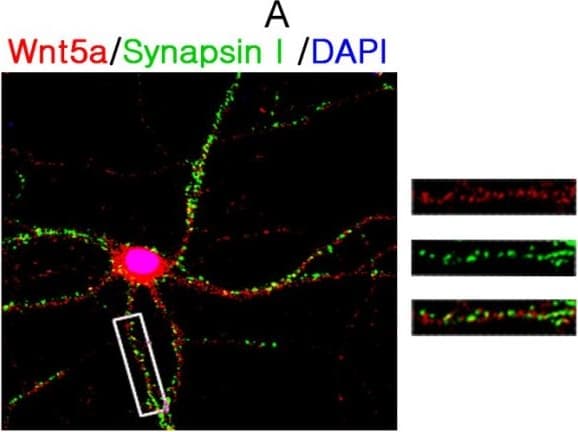
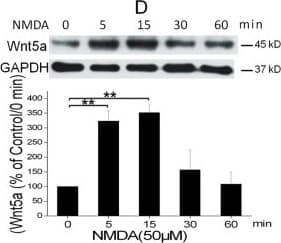
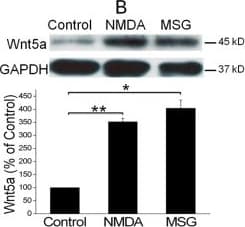
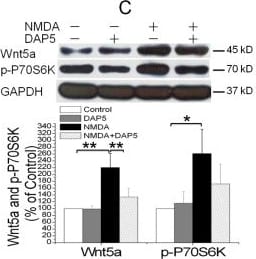
View LargerDetection of Mouse Wnt-5a by Immunocytochemistry/ Immunofluorescence NMDAR activation rapidly increases Wnt5a in cortical cultures. A. Cellular localization of Wnt5a in neurons. Shown are confocal images of primary cortical neurons after double-fluorescent immunostaining with anti-Wnt5a (red) and anti-synapsin I (green) antibodies. The nucleus was stained by DAPI (blue). B. MSG or NMDA stimulation increased Wnt5a protein. Primary cortical neurons (10 DIV) were treated with 10 μΜ MSG or 50 μΜ NMDA for 15 min. Wnt5a protein was detected by Western blotting. Data in the summary graphs (mean ± SEM) were from three independent experiments (*, p < 0.05; **, p < 0.01; One-way ANOVA). C. NMDA receptor-regulated Wnt5a increase. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 100 μΜ DAP5 for 30 min, then incubated with 50 μΜ NMDA for 15 min. Wnt5a and p-P70S6K(included as a marker for translation activation) were detected by Western blotting summarized in the graph (n = 3; *, p < 0.05; **, p < 0.01; One-way ANOVA). D. Dynamic expression of Wnt5a protein after NMDA stimulation. Primary cortical neurons (10 DIV) were treated with 50 μΜ NMDA for 0, 5, 15, 30 and 60 min followed by Western blotting analysis of Wnt5a (n = 3; *, P < 0.05; **, p < 0.01; One-way ANOVA). E. NMDA-induced Wnt5a protein secretion. Primary cortical neurons (10 DIV) were treated with 50 μΜ NMDA for 0, 2, 4, 8, 16 and 32 min, and Wnt5a protein in the media was concentrated and detected on immunoblots. Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View LargerDetection of Mouse Wnt-5a by Western Blot NMDAR activation rapidly increases Wnt5a in cortical cultures. A. Cellular localization of Wnt5a in neurons. Shown are confocal images of primary cortical neurons after double-fluorescent immunostaining with anti-Wnt5a (red) and anti-synapsin I (green) antibodies. The nucleus was stained by DAPI (blue). B. MSG or NMDA stimulation increased Wnt5a protein. Primary cortical neurons (10 DIV) were treated with 10 μΜ MSG or 50 μΜ NMDA for 15 min. Wnt5a protein was detected by Western blotting. Data in the summary graphs (mean ± SEM) were from three independent experiments (*, p < 0.05; **, p < 0.01; One-way ANOVA). C. NMDA receptor-regulated Wnt5a increase. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 100 μΜ DAP5 for 30 min, then incubated with 50 μΜ NMDA for 15 min. Wnt5a and p-P70S6K(included as a marker for translation activation) were detected by Western blotting summarized in the graph (n = 3; *, p < 0.05; **, p < 0.01; One-way ANOVA). D. Dynamic expression of Wnt5a protein after NMDA stimulation. Primary cortical neurons (10 DIV) were treated with 50 μΜ NMDA for 0, 5, 15, 30 and 60 min followed by Western blotting analysis of Wnt5a (n = 3; *, P < 0.05; **, p < 0.01; One-way ANOVA). E. NMDA-induced Wnt5a protein secretion. Primary cortical neurons (10 DIV) were treated with 50 μΜ NMDA for 0, 2, 4, 8, 16 and 32 min, and Wnt5a protein in the media was concentrated and detected on immunoblots. Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
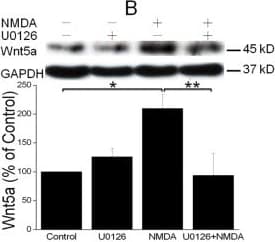
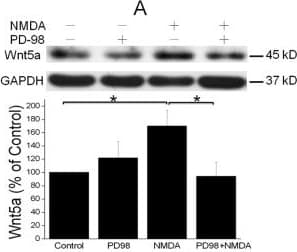
View LargerDetection of Mouse Wnt-5a by Western Blot NMDAR activation stimulates Wnt5a protein synthesis via the MAPK signaling pathway. A. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 20 μM PD98059 (PD98) for 30 min, and then stimulated with 50 μΜ NMDA for 15 min. Wnt5a protein was measured by Western blotting and quantified data were presented in graphs (mean ± SEM; n = 3, *p < 0.05; One-way ANOVA). B. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 20 μM U0126 for 30 min, followed by 50 μΜ NMDA for 15 min. Graphs (mean ± SEM) are from three independent experiments (*, p < 0.05; **, p < 0.01; One-way ANOVA). Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
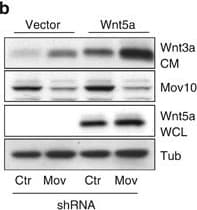
View LargerDetection of Human Wnt-5a by Western Blot Inhibition of Mov10 increases Wnt5a secretion and Ror2-dependent cell invasion. (a) Wnt5a secretion in conditioned media (CM) from UACC903 melanoma cells compared with whole-cell lysate (WCL) from control and Mov10 shRNA-expressing cells. (b) Wnt5a secretion in UACC903 cells expressing a Wnt5a transgene. (c) Wnt3a secretion in UACC903 cells expressing a Wnt3a transgene. (d) Secretion of fibronectin (FN) from UACC903 cells expressing control and Mov10 shRNA. (e) Collagen invasion of WM239A melanoma cells expressing two Mov10 shRNA constructs. (f) Quantification of invasion assay in (a) Student's t-test, compared with shControl, shMov10-1 P=2.29 × 10−11, shMov10-2 P=3.95 × 10−10. (g) Collagen invasion assay of cells expressing Ror2 shRNA and Mov10 shRNA (see Supplementary Figure S2). (h) Quantification of invasion assay in (d). Student's t-test, shMov10+shCtr vs shCtr P=3.07 × 10−11, shMov10+shRor2-1 vs shMov10+shCtr P=9.02 × 10−10, shMov10+shRor2-2 vs shMov10+shCtr P=1.42 × 10−11. Error bars indicate s.d. Image collected and cropped by CiteAb from the following open publication (https://www.nature.com/articles/oncsis201515), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View LargerDetection of Mouse Wnt-5a by Western Blot NMDAR activation stimulates Wnt5a protein synthesis via the MAPK signaling pathway. A. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 20 μM PD98059 (PD98) for 30 min, and then stimulated with 50 μΜ NMDA for 15 min. Wnt5a protein was measured by Western blotting and quantified data were presented in graphs (mean ± SEM; n = 3, *p < 0.05; One-way ANOVA). B. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 20 μM U0126 for 30 min, followed by 50 μΜ NMDA for 15 min. Graphs (mean ± SEM) are from three independent experiments (*, p < 0.05; **, p < 0.01; One-way ANOVA). Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View LargerDetection of Mouse Wnt-5a by Western Blot NMDAR activation rapidly increases Wnt5a in cortical cultures. A. Cellular localization of Wnt5a in neurons. Shown are confocal images of primary cortical neurons after double-fluorescent immunostaining with anti-Wnt5a (red) and anti-synapsin I (green) antibodies. The nucleus was stained by DAPI (blue). B. MSG or NMDA stimulation increased Wnt5a protein. Primary cortical neurons (10 DIV) were treated with 10 μΜ MSG or 50 μΜ NMDA for 15 min. Wnt5a protein was detected by Western blotting. Data in the summary graphs (mean ± SEM) were from three independent experiments (*, p < 0.05; **, p < 0.01; One-way ANOVA). C. NMDA receptor-regulated Wnt5a increase. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 100 μΜ DAP5 for 30 min, then incubated with 50 μΜ NMDA for 15 min. Wnt5a and p-P70S6K(included as a marker for translation activation) were detected by Western blotting summarized in the graph (n = 3; *, p < 0.05; **, p < 0.01; One-way ANOVA). D. Dynamic expression of Wnt5a protein after NMDA stimulation. Primary cortical neurons (10 DIV) were treated with 50 μΜ NMDA for 0, 5, 15, 30 and 60 min followed by Western blotting analysis of Wnt5a (n = 3; *, P < 0.05; **, p < 0.01; One-way ANOVA). E. NMDA-induced Wnt5a protein secretion. Primary cortical neurons (10 DIV) were treated with 50 μΜ NMDA for 0, 2, 4, 8, 16 and 32 min, and Wnt5a protein in the media was concentrated and detected on immunoblots. Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View LargerDetection of Mouse Wnt-5a by Western Blot NMDAR activation rapidly increases Wnt5a in cortical cultures. A. Cellular localization of Wnt5a in neurons. Shown are confocal images of primary cortical neurons after double-fluorescent immunostaining with anti-Wnt5a (red) and anti-synapsin I (green) antibodies. The nucleus was stained by DAPI (blue). B. MSG or NMDA stimulation increased Wnt5a protein. Primary cortical neurons (10 DIV) were treated with 10 μΜ MSG or 50 μΜ NMDA for 15 min. Wnt5a protein was detected by Western blotting. Data in the summary graphs (mean ± SEM) were from three independent experiments (*, p < 0.05; **, p < 0.01; One-way ANOVA). C. NMDA receptor-regulated Wnt5a increase. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 100 μΜ DAP5 for 30 min, then incubated with 50 μΜ NMDA for 15 min. Wnt5a and p-P70S6K(included as a marker for translation activation) were detected by Western blotting summarized in the graph (n = 3; *, p < 0.05; **, p < 0.01; One-way ANOVA). D. Dynamic expression of Wnt5a protein after NMDA stimulation. Primary cortical neurons (10 DIV) were treated with 50 μΜ NMDA for 0, 5, 15, 30 and 60 min followed by Western blotting analysis of Wnt5a (n = 3; *, P < 0.05; **, p < 0.01; One-way ANOVA). E. NMDA-induced Wnt5a protein secretion. Primary cortical neurons (10 DIV) were treated with 50 μΜ NMDA for 0, 2, 4, 8, 16 and 32 min, and Wnt5a protein in the media was concentrated and detected on immunoblots. Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
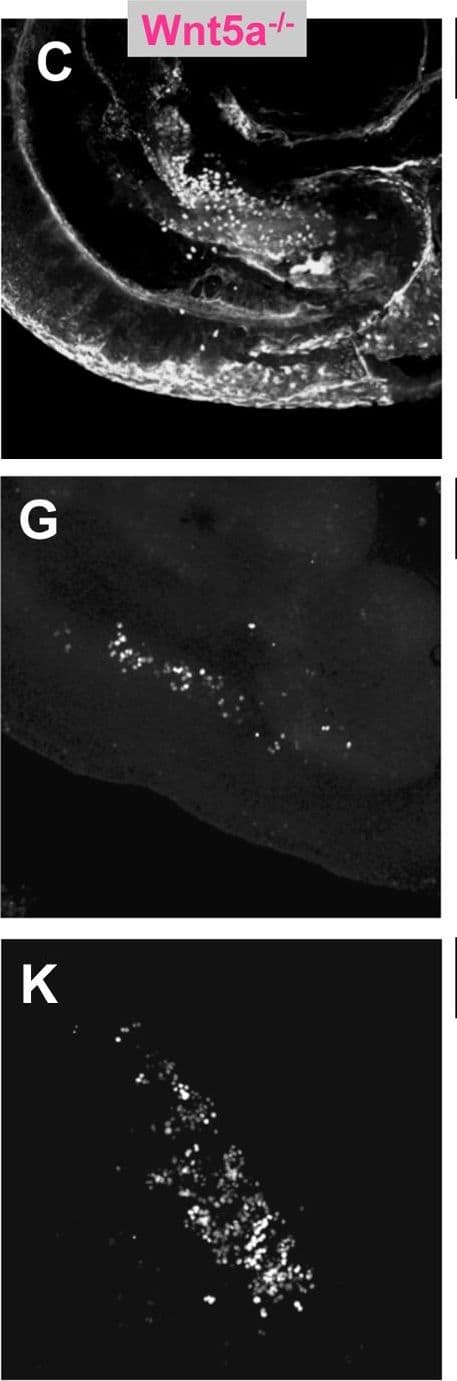
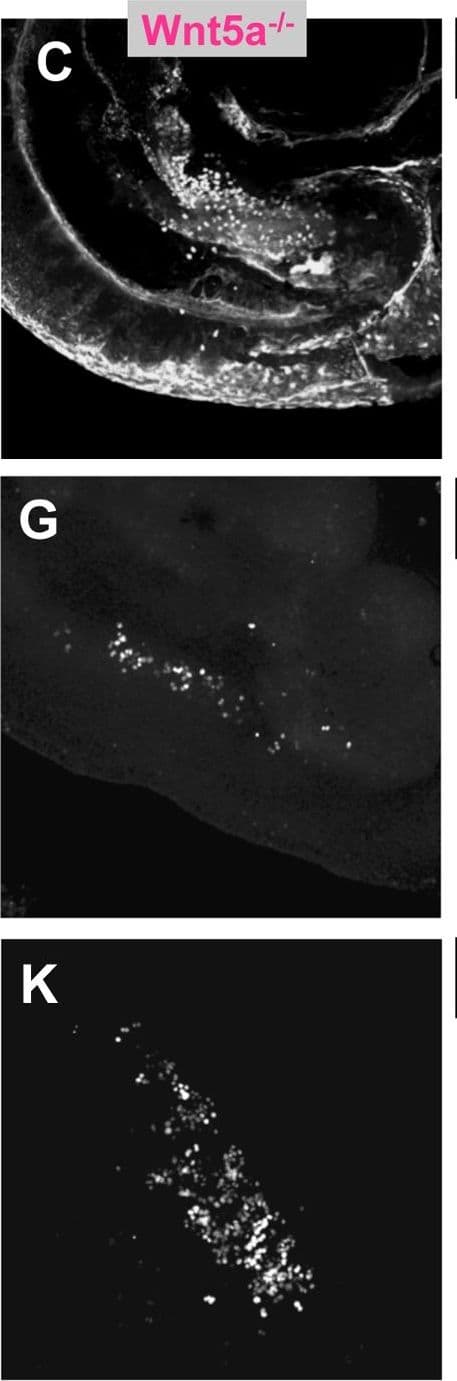
View LargerDetection of Mouse Wnt-5a by Immunohistochemistry PGC depletion in Ror2 and Wnt5a mutants.(A–C) PGCs were visualized by wholemount SSEA1 immunostaining with SSEA1 antibody in e10.25 WT, Ror2, and Wnt5a embryos. (E–G) Gonadal ridges from e11.5 stained with GCNA, and (I–K) e12.5 male gonads stained with GCNA antibody. The caudal end is down in all images. (D, H, L) Quantification of PGCs in the entire e10.25 embryo, e11.5 and e12.5 gonads from confocal stacks, with individuals denoted as WT (diamond), Ror2Y324C mutants (triangle), and Wnt5a null (circle), and means indicated as bars. Consistent with appearances, a significant reduction of PGCs was observed in Wnt5a and from e11.5 onward in Ror2Y324C. We noted no significant difference in the number of PGCs between XX and XY gonads at e12.5 (not shown). Results of the Student's t-test are indicated, * p<0.05, **, p<0.01, ***p<0.001. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pgen.1002428), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
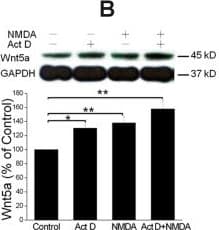
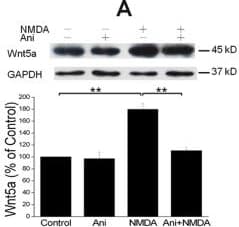
View LargerDetection of Mouse Wnt-5a by Western Blot NMDAR-elicited Wnt5a increase requires translation but not transcription. A. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 20 μΜ anisomycin for 30 min, and then incubated with 50 μΜ NMDA for 15 min, followed by Wnt5a immunoblotting. The Graph is a summary of three independent experiments (**, p < 0.01; One-way ANOVA). B. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 20 μΜ actinomycin D for 30 min, followed by addition of 50 μΜ NMDA for 15 min. Wnt5a protein was detected by Western blotting and quantified. The Graph is a summary of four independent experiments (*, p < 0.05; **, p < 0.01; One-way ANOVA). C. Primary cortical neurons (10 DIV) were treated with vehicle (Control) or 50 μΜ NMDA for 15 min. Wnt5a mRNA was quantified by Real-time RT-PCR (qPCR). The summary graph is from three independent experiments (40 cycles, CT values: 25.1 ± 0.5/control vs. 25.6 ± 0.3/NMDA; p > 0.05; two-tailed Student's tests). D. Melt curve of Wnt5a qPCR on control cells. The melt curve on NMDA-stimulated cells was similar (not shown). E. RT-PCR results of Wnt5a in control and NMDA-treated cells. Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View LargerDetection of Mouse Wnt-5a by Western Blot NMDAR-elicited Wnt5a increase requires translation but not transcription. A. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 20 μΜ anisomycin for 30 min, and then incubated with 50 μΜ NMDA for 15 min, followed by Wnt5a immunoblotting. The Graph is a summary of three independent experiments (**, p < 0.01; One-way ANOVA). B. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 20 μΜ actinomycin D for 30 min, followed by addition of 50 μΜ NMDA for 15 min. Wnt5a protein was detected by Western blotting and quantified. The Graph is a summary of four independent experiments (*, p < 0.05; **, p < 0.01; One-way ANOVA). C. Primary cortical neurons (10 DIV) were treated with vehicle (Control) or 50 μΜ NMDA for 15 min. Wnt5a mRNA was quantified by Real-time RT-PCR (qPCR). The summary graph is from three independent experiments (40 cycles, CT values: 25.1 ± 0.5/control vs. 25.6 ± 0.3/NMDA; p > 0.05; two-tailed Student's tests). D. Melt curve of Wnt5a qPCR on control cells. The melt curve on NMDA-stimulated cells was similar (not shown). E. RT-PCR results of Wnt5a in control and NMDA-treated cells. Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
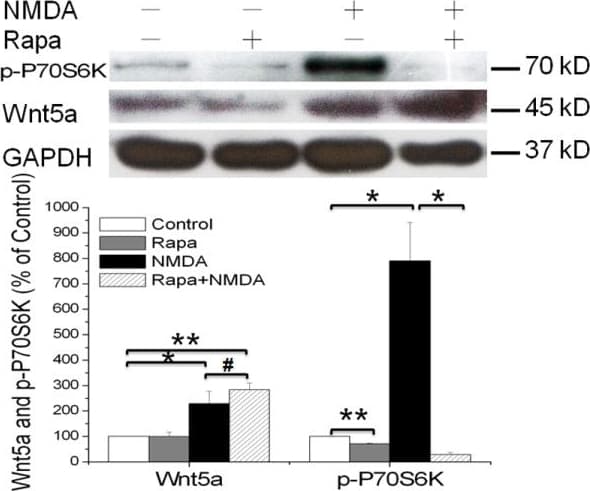
View LargerDetection of Mouse Wnt-5a by Western Blot mTOR signaling pathway is not required for the NMDAR-dependent Wnt5a protein synthesis. Primary cortical neurons (10 DIV) were pre-treated with vehicle (Control) or 25nΜ Rapamycin for 30 min, followed by addition of 50 μΜ NMDA for 15 min. Western blotting analysis of Wnt5a and phosphor-P70S6K proteins were performed. Graphs (mean ± SEM) are from four independent experiments (*, p < 0.05; **, p < 0.01; #, p > 0.05; One-way ANOVA). Image collected and cropped by CiteAb from the following open publication (https://molecularbrain.biomedcentral.com/articles/10.1186/1756-6606-5-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View LargerDetection of Mouse Wnt-5a by Immunohistochemistry PGC depletion in Ror2 and Wnt5a mutants.(A–C) PGCs were visualized by wholemount SSEA1 immunostaining with SSEA1 antibody in e10.25 WT, Ror2, and Wnt5a embryos. (E–G) Gonadal ridges from e11.5 stained with GCNA, and (I–K) e12.5 male gonads stained with GCNA antibody. The caudal end is down in all images. (D, H, L) Quantification of PGCs in the entire e10.25 embryo, e11.5 and e12.5 gonads from confocal stacks, with individuals denoted as WT (diamond), Ror2Y324C mutants (triangle), and Wnt5a null (circle), and means indicated as bars. Consistent with appearances, a significant reduction of PGCs was observed in Wnt5a and from e11.5 onward in Ror2Y324C. We noted no significant difference in the number of PGCs between XX and XY gonads at e12.5 (not shown). Results of the Student's t-test are indicated, * p<0.05, **, p<0.01, ***p<0.001. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pgen.1002428), licensed under a CC-BY license. Not internally tested by R&D Systems.
Mouse/Rat Wnt-5a Antibody Summary
Gln254-Cys334
Accession # P22725
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Immunohistochemistry(5-15 µg/mL)


Background: Wnt-5a
Wnt proteins are secreted glycoproteins that contain a conserved pattern of 23-24 cysteine residues. Wnts play critical roles in both carcinogenesis and embryonic development for a variety of organisms. Wnts bind to receptors of the Frizzled family, sometimes in conjunction with other membrane-associated proteins such as LRPs or proteoglycans. Downstream effects of Wnt signaling occur through different intracellular components, depending on which pathway is activated. Three pathways have been characterized: the canonical Wnt/ beta -catenin pathway, the Wnt/Ca2+ pathway, and the planar cell polarity (1, 2).
Wnt-5a is part of the subgroup of Wnts that are not axis-inducing in Xenopus embryos and do not transform C57MG mammary epithelial cells. This subgroup is also implicated in the Wnt/Ca2+ pathway, playing roles in cell movements and cell adhesion (3). This non-canonical Wnt pathway can inhibit canonical Wnt/ beta -catenin signaling. In Wnt-5a deficient mouse embryos, beta -catenin accumulates in the limb bud suggesting that Wnt-5a normally promotes degradation of beta -catenin (4). Likewise, in Xenopus embryos Wnt-5a antagonizes the ability of the canonical Wnt subgroup to induce a secondary axis (5). Wnt-5a is implicated in various types of cancer and has complex roles. It acts as a tumor suppressor for mammary, B-cell, colon, and uroepithelial cancer cells but is up-regulated in melanomas, where expression levels correlate with severity of metastasis (3). Furthermore, aberrant Wnt-5a signaling results in other diseases such as rheumatoid arthritis (6). Like other developmental growth factors Wnt-5a has diverse roles in development. They are too numerous to enunciate here, as functions span from early anterior-posterior development and gastrulation movements to maintaining hematopoietic stem cell population, lung morphogenesis, and limb outgrowth. Mouse and human Wnt-5a share 97% amino acid identity.
- Miller, J.R. (2002) Genome Biol. 3:3001.
- Roelink, H. and R. Nusse (1991) Genes Dev. 5:381.
- Veeman, M.T. et al. (2003) Developmental Cell 5:367.
- Topol, L. et al. (2003) J. Cell Biol 162:899.
- Torres, M. et al. (1996) J. Cell Biol. 133:1123.
- Sen, M. et al. (2001) Arthritis & Rheumatism 44:772.


Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
参考图片
Wnt‑5a in Mouse Embryonic Rib. Wnt‑5a was detected in immersion fixed paraffin-embedded sections of mouse embryonic rib using 15 µg/mL Mouse/Rat Wnt‑5a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF645) overnight at 4 °C. Tissue was stained with the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
Detection of Mouse Wnt‑5a by Western Blot. Western blot shows lysates of mouse brain and lactating mammary tissue. PVDF membrane was probed with 1 µg/mL of Mouse/Rat Wnt‑5a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF645) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF019). A specific band was detected for Wnt‑5a at approximately 45 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 8.
Wnt‑5a in Mouse Embryo. Wnt‑5a was detected in immersion fixed frozen sections of mouse embryo using Mouse/Rat Wnt‑5a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF645) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.